ISSN: 2320-5407 Int. J. Adv. Res. 5(9), 945-957 RESEARCH ARTICLE
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Molecular Phylogenetic Studies in the Genus Amanita
1170 Molecular phylogenetic studies in the genus Amanita I5ichael Weiß, Zhu-Liang Yang, and Franz Oberwinkler Abstracl A group of 49 Amanita species that had been thoroughly examined morphologically and amtomically was analyzed by DNA sequence compadson to estimate natural groups and phylogenetic rclationships within the genus. Nuclear DNA sequences coding for a part of the ribosomal large subunit were determined and evaluated using neighbor-joining with bootstrap analysis, parsimony analysis, conditional clustering, and maximum likelihood methods, Sections Amanita, Caesarea, Vaginatae, Validae, Phalloideae, and Amidella were substantially confirmed as monophyletic groups, while the monophyly of section Lepidell.t remained unclear. Branching topologies between and within sections could also pafiially be derived. Stbgenera Amanita an'd Lepidella were not supported. The Mappae group was included in section Validae. Grouping hypotheses obtained by DNA analyses are discussed in relation to the distribution of morphological and anatomical chamcters in the studied species. Key words: fungi, basidiomycetes phylogeny, Agarrcales, Amanita systematics, large subunit rDNA, 28S. R6sum6 : A partir d'un groupe de 49 esp,ces d'Amanita prdalablement examinees morphologiquement et anatomiquement, les auteurs ont utilisd la comparaison des s€quences d'ADN pour ddfinir les groupes naturels et les relations phylog6ndtiques de ce genre. Les sdquences de I'ADN nucl6aire codant pour une partie de la grande sous-unit6 ribosomale ont 6t6 ddterminEes et €valu6es en utilisant l'analyse par liaison en lacet avec le voisin (neighbor-joining with bootstrap), l'analyse en parcimonie, le rcgroupement conditionnel et les m€thodes de ressemblance maximale. Les rdsultats confirment substantiellement les sections Afiarira, Caesarea, Uaqinatae, Ualidae, Phalloideae et Amidella, comme groupes monophyldtiques, alors que la monophylie de la section Lepidella demerxe obscure. -

Major Clades of Agaricales: a Multilocus Phylogenetic Overview
Mycologia, 98(6), 2006, pp. 982–995. # 2006 by The Mycological Society of America, Lawrence, KS 66044-8897 Major clades of Agaricales: a multilocus phylogenetic overview P. Brandon Matheny1 Duur K. Aanen Judd M. Curtis Laboratory of Genetics, Arboretumlaan 4, 6703 BD, Biology Department, Clark University, 950 Main Street, Wageningen, The Netherlands Worcester, Massachusetts, 01610 Matthew DeNitis Vale´rie Hofstetter 127 Harrington Way, Worcester, Massachusetts 01604 Department of Biology, Box 90338, Duke University, Durham, North Carolina 27708 Graciela M. Daniele Instituto Multidisciplinario de Biologı´a Vegetal, M. Catherine Aime CONICET-Universidad Nacional de Co´rdoba, Casilla USDA-ARS, Systematic Botany and Mycology de Correo 495, 5000 Co´rdoba, Argentina Laboratory, Room 304, Building 011A, 10300 Baltimore Avenue, Beltsville, Maryland 20705-2350 Dennis E. Desjardin Department of Biology, San Francisco State University, Jean-Marc Moncalvo San Francisco, California 94132 Centre for Biodiversity and Conservation Biology, Royal Ontario Museum and Department of Botany, University Bradley R. Kropp of Toronto, Toronto, Ontario, M5S 2C6 Canada Department of Biology, Utah State University, Logan, Utah 84322 Zai-Wei Ge Zhu-Liang Yang Lorelei L. Norvell Kunming Institute of Botany, Chinese Academy of Pacific Northwest Mycology Service, 6720 NW Skyline Sciences, Kunming 650204, P.R. China Boulevard, Portland, Oregon 97229-1309 Jason C. Slot Andrew Parker Biology Department, Clark University, 950 Main Street, 127 Raven Way, Metaline Falls, Washington 99153- Worcester, Massachusetts, 01609 9720 Joseph F. Ammirati Else C. Vellinga University of Washington, Biology Department, Box Department of Plant and Microbial Biology, 111 355325, Seattle, Washington 98195 Koshland Hall, University of California, Berkeley, California 94720-3102 Timothy J. -

Cultural Characterization and Chlamydospore Function of the Ganodermataceae Present in the Eastern United States
Mycologia ISSN: 0027-5514 (Print) 1557-2536 (Online) Journal homepage: https://www.tandfonline.com/loi/umyc20 Cultural characterization and chlamydospore function of the Ganodermataceae present in the eastern United States Andrew L. Loyd, Eric R. Linder, Matthew E. Smith, Robert A. Blanchette & Jason A. Smith To cite this article: Andrew L. Loyd, Eric R. Linder, Matthew E. Smith, Robert A. Blanchette & Jason A. Smith (2019): Cultural characterization and chlamydospore function of the Ganodermataceae present in the eastern United States, Mycologia To link to this article: https://doi.org/10.1080/00275514.2018.1543509 View supplementary material Published online: 24 Jan 2019. Submit your article to this journal View Crossmark data Full Terms & Conditions of access and use can be found at https://www.tandfonline.com/action/journalInformation?journalCode=umyc20 MYCOLOGIA https://doi.org/10.1080/00275514.2018.1543509 Cultural characterization and chlamydospore function of the Ganodermataceae present in the eastern United States Andrew L. Loyd a, Eric R. Lindera, Matthew E. Smith b, Robert A. Blanchettec, and Jason A. Smitha aSchool of Forest Resources and Conservation, University of Florida, Gainesville, Florida 32611; bDepartment of Plant Pathology, University of Florida, Gainesville, Florida 32611; cDepartment of Plant Pathology, University of Minnesota, St. Paul, Minnesota 55108 ABSTRACT ARTICLE HISTORY The cultural characteristics of fungi can provide useful information for studying the biology and Received 7 Feburary 2018 ecology of a group of closely related species, but these features are often overlooked in the order Accepted 30 October 2018 Polyporales. Optimal temperature and growth rate data can also be of utility for strain selection of KEYWORDS cultivated fungi such as reishi (i.e., laccate Ganoderma species) and potential novel management Chlamydospores; tactics (e.g., solarization) for butt rot diseases caused by Ganoderma species. -

AMATOXIN MUSHROOM POISONING in NORTH AMERICA 2015-2016 by Michael W
VOLUME 57: 4 JULY-AUGUST 2017 www.namyco.org AMATOXIN MUSHROOM POISONING IN NORTH AMERICA 2015-2016 By Michael W. Beug: Chair, NAMA Toxicology Committee Assessing the degree of amatoxin mushroom poisoning in North America is very challenging. Understanding the potential for various treatment practices is even more daunting. Although I have been studying mushroom poisoning for 45 years now, my own views on potential best treatment practices are still evolving. While my training in enzyme kinetics helps me understand the literature about amatoxin poisoning treatments, my lack of medical training limits me. Fortunately, critical comments from six different medical doctors have been incorporated in this article. All six, each concerned about different aspects in early drafts, returned me to the peer reviewed scientific literature for additional reading. There remains no known specific antidote for amatoxin poisoning. There have not been any gold standard double-blind placebo controlled studies. There never can be. When dealing with a potentially deadly poisoning (where in many non-western countries the amatoxin fatality rate exceeds 50%) treating of half of all poisoning patients with a placebo would be unethical. Using amatoxins on large animals to test new treatments (theoretically a great alternative) has ethical constraints on the experimental design that would most likely obscure the answers researchers sought. We must thus make our best judgement based on analysis of past cases. Although that number is now large enough that we can make some good assumptions, differences of interpretation will continue. Nonetheless, we may be on the cusp of reaching some agreement. Towards that end, I have contacted several Poison Centers and NAMA will be working with the Centers for Disease Control (CDC). -

Phylogenetic Classification of Trametes
TAXON 60 (6) • December 2011: 1567–1583 Justo & Hibbett • Phylogenetic classification of Trametes SYSTEMATICS AND PHYLOGENY Phylogenetic classification of Trametes (Basidiomycota, Polyporales) based on a five-marker dataset Alfredo Justo & David S. Hibbett Clark University, Biology Department, 950 Main St., Worcester, Massachusetts 01610, U.S.A. Author for correspondence: Alfredo Justo, [email protected] Abstract: The phylogeny of Trametes and related genera was studied using molecular data from ribosomal markers (nLSU, ITS) and protein-coding genes (RPB1, RPB2, TEF1-alpha) and consequences for the taxonomy and nomenclature of this group were considered. Separate datasets with rDNA data only, single datasets for each of the protein-coding genes, and a combined five-marker dataset were analyzed. Molecular analyses recover a strongly supported trametoid clade that includes most of Trametes species (including the type T. suaveolens, the T. versicolor group, and mainly tropical species such as T. maxima and T. cubensis) together with species of Lenzites and Pycnoporus and Coriolopsis polyzona. Our data confirm the positions of Trametes cervina (= Trametopsis cervina) in the phlebioid clade and of Trametes trogii (= Coriolopsis trogii) outside the trametoid clade, closely related to Coriolopsis gallica. The genus Coriolopsis, as currently defined, is polyphyletic, with the type species as part of the trametoid clade and at least two additional lineages occurring in the core polyporoid clade. In view of these results the use of a single generic name (Trametes) for the trametoid clade is considered to be the best taxonomic and nomenclatural option as the morphological concept of Trametes would remain almost unchanged, few new nomenclatural combinations would be necessary, and the classification of additional species (i.e., not yet described and/or sampled for mo- lecular data) in Trametes based on morphological characters alone will still be possible. -

Download Download
LITERATURE UPDATE FOR TEXAS FLESHY BASIDIOMYCOTA WITH NEW VOUCHERED RECORDS FOR SOUTHEAST TEXAS David P. Lewis Clark L. Ovrebo N. Jay Justice 262 CR 3062 Department of Biology 16055 Michelle Drive Newton, Texas 75966, U.S.A. University of Central Oklahoma Alexander, Arkansas 72002, U.S.A. [email protected] Edmond, Oklahoma 73034, U.S.A. [email protected] [email protected] ABSTRACT This is a second paper documenting the literature records for Texas fleshy basidiomycetous fungi and includes both older literature and recently published papers. We report 80 literature articles which include 14 new taxa described from Texas. We also report on 120 new records of fleshy basdiomycetous fungi collected primarily from southeast Texas. RESUMEN Este es un segundo artículo que documenta el registro de nuevas especies de hongos carnosos basidiomicetos, incluyendo artículos antiguos y recientes. Reportamos 80 artículos científicamente relacionados con estas especies que incluyen 14 taxones con holotipos en Texas. Así mismo, reportamos unos 120 nuevos registros de hongos carnosos basidiomicetos recolectados primordialmente en al sureste de Texas. PART I—MYCOLOGICAL LITERATURE ON TEXAS FLESHY BASIDIOMYCOTA Lewis and Ovrebo (2009) previously reported on literature for Texas fleshy Basidiomycota and also listed new vouchered records for Texas of that group. Presented here is an update to the listing which includes literature published since 2009 and also includes older references that we previously had not uncovered. The authors’ primary research interests center around gilled mushrooms and boletes so perhaps the list that follows is most complete for the fungi of these groups. We have, however, attempted to locate references for all fleshy basidio- mycetous fungi. -

A Synopsis of the Saddle Fungi (Helvella: Ascomycota) in Europe – Species Delimitation, Taxonomy and Typification
Persoonia 39, 2017: 201–253 ISSN (Online) 1878-9080 www.ingentaconnect.com/content/nhn/pimj RESEARCH ARTICLE https://doi.org/10.3767/persoonia.2017.39.09 A synopsis of the saddle fungi (Helvella: Ascomycota) in Europe – species delimitation, taxonomy and typification I. Skrede1,*, T. Carlsen1, T. Schumacher1 Key words Abstract Helvella is a widespread, speciose genus of large apothecial ascomycetes (Pezizomycete: Pezizales) that are found in terrestrial biomes of the Northern and Southern Hemispheres. This study represents a beginning on molecular phylogeny assessing species limits and applying correct names for Helvella species based on type material and specimens in the Pezizales university herbaria (fungaria) of Copenhagen (C), Harvard (FH) and Oslo (O). We use morphology and phylogenetic systematics evidence from four loci – heat shock protein 90 (hsp), translation elongation factor alpha (tef), RNA polymerase II (rpb2) and the nuclear large subunit ribosomal DNA (LSU) – to assess species boundaries in an expanded sample of Helvella specimens from Europe. We combine the morphological and phylogenetic information from 55 Helvella species from Europe with a small sample of Helvella species from other regions of the world. Little intraspecific variation was detected within the species using these molecular markers; hsp and rpb2 markers provided useful barcodes for species delimitation in this genus, while LSU provided more variable resolution among the pertinent species. We discuss typification issues and identify molecular characteristics for 55 European Helvella species, designate neo- and epitypes for 30 species, and describe seven Helvella species new to science, i.e., H. alpicola, H. alpina, H. carnosa, H. danica, H. nannfeldtii, H. pubescens and H. -

Bioluminescence in Mushroom and Its Application Potentials
Nigerian Journal of Science and Environment, Vol. 14 (1) (2016) BIOLUMINESCENCE IN MUSHROOM AND ITS APPLICATION POTENTIALS Ilondu, E. M.* and Okiti, A. A. Department of Botany, Faculty of Science, Delta State University, Abraka, Nigeria. *Corresponding author. E-mail: [email protected]. Tel: 2348036758249. ABSTRACT Bioluminescence is a biological process through which light is produced and emitted by a living organism resulting from a chemical reaction within the body of the organism. The mechanism behind this phenomenon is an oxygen-dependent reaction involving substrates generally termed luciferin, which is catalyzed by one or more of an assortment of unrelated enzyme called luciferases. The history of bioluminescence in fungi can be traced far back to 382 B.C. when it was first noted by Aristotle in his early writings. It is the nature of bioluminescent mushrooms to emit a greenish light at certain stages in their life cycle and this light has a maximum wavelength range of 520-530 nm. Luminescence in mushroom has been hypothesized to attract invertebrates that aids in spore dispersal and testing for pollutants (ions of mercury) in water supply. The metabolites from luminescent mushrooms are effectively bioactive in anti-moulds, anti-bacteria, anti-virus, especially in inhibiting the growth of cancer cell and very useful in areas of biology, biotechnology and medicine as luminescent markers for developing new luminescent microanalysis methods. Luminescent mushroom is a novel area of research in the world which is beneficial to mankind especially with regards to environmental pollution monitoring and biomedical applications. Bioluminescence in fungi is a beautiful phenomenon to observe which should be of interest to Scientists of all endeavors. -

The Phylogeny of Plant and Animal Pathogens in the Ascomycota
Physiological and Molecular Plant Pathology (2001) 59, 165±187 doi:10.1006/pmpp.2001.0355, available online at http://www.idealibrary.com on MINI-REVIEW The phylogeny of plant and animal pathogens in the Ascomycota MARY L. BERBEE* Department of Botany, University of British Columbia, 6270 University Blvd, Vancouver, BC V6T 1Z4, Canada (Accepted for publication August 2001) What makes a fungus pathogenic? In this review, phylogenetic inference is used to speculate on the evolution of plant and animal pathogens in the fungal Phylum Ascomycota. A phylogeny is presented using 297 18S ribosomal DNA sequences from GenBank and it is shown that most known plant pathogens are concentrated in four classes in the Ascomycota. Animal pathogens are also concentrated, but in two ascomycete classes that contain few, if any, plant pathogens. Rather than appearing as a constant character of a class, the ability to cause disease in plants and animals was gained and lost repeatedly. The genes that code for some traits involved in pathogenicity or virulence have been cloned and characterized, and so the evolutionary relationships of a few of the genes for enzymes and toxins known to play roles in diseases were explored. In general, these genes are too narrowly distributed and too recent in origin to explain the broad patterns of origin of pathogens. Co-evolution could potentially be part of an explanation for phylogenetic patterns of pathogenesis. Robust phylogenies not only of the fungi, but also of host plants and animals are becoming available, allowing for critical analysis of the nature of co-evolutionary warfare. Host animals, particularly human hosts have had little obvious eect on fungal evolution and most cases of fungal disease in humans appear to represent an evolutionary dead end for the fungus. -
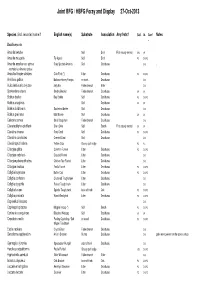
Joint BFG / HBFG Foray and Display 27-Oct-2013
Joint BFG / HBFG Foray and Display 27-Oct-2013 Species (incl. recorded name if English name(s) Substrate Association Any firsts? Coll. Id. Conf Notes . Basidiomycota Amanita betulae Soil Birch First county record SH3 SK Amanita muscaria Fly Agaric Soil Birch PC DJSPC Amanita excelsa var. spissa Grey Spotted Amanita Soil Deciduous DJS . recorded as Amanita spissa Ampulloclitocybe clavipes Club Foot (*) Litter Deciduous PC DJSPC . Armillaria gallica Bulbous Honey Fungus on roots Deciduous DJS Auricularia auricula-judae Jelly Ear Fallen branch Elder DJS Bjerkandera adusta Smoky Bracket Fallen branch Deciduous SK SK Boletus badius Bay Bolete Soil Deciduous PC DJSPC Boletus cisalpinus Soil Deciduous SK SK Boletus luridiformis Scarletina Bolete Soil Deciduous DJS Boletus pruinatus Matt Bolete Soil Deciduous SK SK Calocera cornea Small Stagshorn Fallen branch Deciduous DJS Clavariadelphus pistillaris Giant Club Soil Beech First county record SK SK Clavulina cinerea Grey Coral Soil Deciduous PC DJSPC Clavulina coralloides Crested Coral Soil Deciduous DJS Clavulinopsis helvola Yellow Club Grassy path edge PC PC Clitocybe gibba Common Funnel Litter Deciduous PC DJSPC Clitocybe nebularis Clouded Funnel Litter Deciduous DJS Clitocybe phaeophthalma Chicken Run Funnel Litter Deciduous DJS Clitocybe rivulosa Fool's Funnel Litter Deciduous PC DJSPC Collybia butyracea Butter Cap Litter Deciduous PC DJSPC Collybia confluens Clustered Toughshank Litter Deciduous DJS Collybia dryophila Russet Toughshank Litter Deciduous DJS Collybia fusipes Spindle Toughshank -

2 the Numbers Behind Mushroom Biodiversity
15 2 The Numbers Behind Mushroom Biodiversity Anabela Martins Polytechnic Institute of Bragança, School of Agriculture (IPB-ESA), Portugal 2.1 Origin and Diversity of Fungi Fungi are difficult to preserve and fossilize and due to the poor preservation of most fungal structures, it has been difficult to interpret the fossil record of fungi. Hyphae, the vegetative bodies of fungi, bear few distinctive morphological characteristicss, and organisms as diverse as cyanobacteria, eukaryotic algal groups, and oomycetes can easily be mistaken for them (Taylor & Taylor 1993). Fossils provide minimum ages for divergences and genetic lineages can be much older than even the oldest fossil representative found. According to Berbee and Taylor (2010), molecular clocks (conversion of molecular changes into geological time) calibrated by fossils are the only available tools to estimate timing of evolutionary events in fossil‐poor groups, such as fungi. The arbuscular mycorrhizal symbiotic fungi from the division Glomeromycota, gen- erally accepted as the phylogenetic sister clade to the Ascomycota and Basidiomycota, have left the most ancient fossils in the Rhynie Chert of Aberdeenshire in the north of Scotland (400 million years old). The Glomeromycota and several other fungi have been found associated with the preserved tissues of early vascular plants (Taylor et al. 2004a). Fossil spores from these shallow marine sediments from the Ordovician that closely resemble Glomeromycota spores and finely branched hyphae arbuscules within plant cells were clearly preserved in cells of stems of a 400 Ma primitive land plant, Aglaophyton, from Rhynie chert 455–460 Ma in age (Redecker et al. 2000; Remy et al. 1994) and from roots from the Triassic (250–199 Ma) (Berbee & Taylor 2010; Stubblefield et al. -

Boletes from Belize and the Dominican Republic
Fungal Diversity Boletes from Belize and the Dominican Republic Beatriz Ortiz-Santana1*, D. Jean Lodge2, Timothy J. Baroni3 and Ernst E. Both4 1Center for Forest Mycology Research, Northern Research Station, USDA-FS, Forest Products Laboratory, One Gifford Pinchot Drive, Madison, Wisconsin 53726-2398, USA 2Center for Forest Mycology Research, Northern Research Station, USDA-FS, PO Box 1377, Luquillo, Puerto Rico 00773-1377, USA 3Department of Biological Sciences, PO Box 2000, SUNY-College at Cortland, Cortland, New York 13045, USA 4Buffalo Museum of Science, 1020 Humboldt Parkway, Buffalo, New York 14211, USA Ortiz-Santana, B., Lodge, D.J., Baroni, T.J. and Both, E.E. (2007). Boletes from Belize and the Dominican Republic. Fungal Diversity 27: 247-416. This paper presents results of surveys of stipitate-pileate Boletales in Belize and the Dominican Republic. A key to the Boletales from Belize and the Dominican Republic is provided, followed by descriptions, drawings of the micro-structures and photographs of each identified species. Approximately 456 collections from Belize and 222 from the Dominican Republic were studied comprising 58 species of boletes, greatly augmenting the knowledge of the diversity of this group in the Caribbean Basin. A total of 52 species in 14 genera were identified from Belize, including 14 new species. Twenty-nine of the previously described species are new records for Belize and 11 are new for Central America. In the Dominican Republic, 14 species in 7 genera were found, including 4 new species, with one of these new species also occurring in Belize, i.e. Retiboletus vinaceipes. Only one of the previously described species found in the Dominican Republic is a new record for Hispaniola and the Caribbean.