Review of Phytophthora Root Rot of Chestnuts
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CHESTNUT (CASTANEA Spp.) CULTIVAR EVALUATION for COMMERCIAL CHESTNUT PRODUCTION
CHESTNUT (CASTANEA spp.) CULTIVAR EVALUATION FOR COMMERCIAL CHESTNUT PRODUCTION IN HAMILTON COUNTY, TENNESSEE By Ana Maria Metaxas Approved: James Hill Craddock Jennifer Boyd Professor of Biological Sciences Assistant Professor of Biological and Environmental Sciences (Director of Thesis) (Committee Member) Gregory Reighard Jeffery Elwell Professor of Horticulture Dean, College of Arts and Sciences (Committee Member) A. Jerald Ainsworth Dean of the Graduate School CHESTNUT (CASTANEA spp.) CULTIVAR EVALUATION FOR COMMERCIAL CHESTNUT PRODUCTION IN HAMILTON COUNTY, TENNESSEE by Ana Maria Metaxas A Thesis Submitted to the Faculty of the University of Tennessee at Chattanooga in Partial Fulfillment of the Requirements for the Degree of Master of Science in Environmental Science May 2013 ii ABSTRACT Chestnut cultivars were evaluated for their commercial applicability under the environmental conditions in Hamilton County, TN at 35°13ꞌ 45ꞌꞌ N 85° 00ꞌ 03.97ꞌꞌ W elevation 230 meters. In 2003 and 2004, 534 trees were planted, representing 64 different cultivars, varieties, and species. Twenty trees from each of 20 different cultivars were planted as five-tree plots in a randomized complete block design in four blocks of 100 trees each, amounting to 400 trees. The remaining 44 chestnut cultivars, varieties, and species served as a germplasm collection. These were planted in guard rows surrounding the four blocks in completely randomized, single-tree plots. In the analysis, we investigated our collection predominantly with the aim to: 1) discover the degree of acclimation of grower- recommended cultivars to southeastern Tennessee climatic conditions and 2) ascertain the cultivars’ ability to survive in the area with Cryphonectria parasitica and other chestnut diseases and pests present. -

The Origin and Distribution of Phytophthora Cinnamomi
THE ORIGIN AND DISTRIBUTION OF PHYTOPHTHORA CINNAMOMI RANDS IN AUSTRALIAN NATIVE PLANT COMMUNITIES AND THE SIGNIFICANCE OF ITS ASSOCIATION WITH PARTICULAR PLANT SPECIES By B. H. PRATT* and W. A. HEATHER* [Manuscript received 23 October 1972] Abstract The origin, distribution, and disease association of P. cinnamomi in native plant communities in Australia has been examined. The fungus was isolated from the root zones of 31 plant genera in 16 families and is widespread throughout eastern and southern Australia and south-western Western Australia. Although the fungus is associated with disease in native plant communities it is also present in apparently non-diseased communities. Disease occurs usually only in environments disturbed by man, probably as a result of increase in population or activity of pre-existing fungal populations. The widespread distribution of P. cinnamomi in native vegetation in Australia, its occurrence in remote, undisturbed areas, and the apparent balance it has achieved with plant species of differing susceptibility to disease in some natural, undisturbed areas suggests that the fungus is likely to be indigenous to eastern Australia. Further, it may be partly responsible for the localized distribution of some plant species. I. INTRODUCTION The soil-borne fungus Phytophthora cinnamomi Rands is widely distributed in Australia, in horticultural plantings (Zentmyer and Thorn 1967; Pratt and Wrigley 1970; as well as personal communications from the New South Wales Department of Agriculture, Tasmanian Department of Agriculture, Victorian Department of Agriculture, and the Queensland Department of Primary Industries), and in conifer nurseries and plantings (Oxenham and Winks 1963; Bertus 1968; and personal communications from the New South Wales Forestry Commission, and the Queens land Department of Forestry). -

Biodiversity
Biodiversity KEY5 FACTS as hunting), as pasture grasses or as aquarium species Introduced (in the case of some marine species). They have also • Introduced species are been introduced accidentally, such as in shipments of recognised as a leading Species imported grain or in ballast water. cause of biodiversity loss Introduced plants, or weeds, can invade and world-wide. compete with native plant species for space, light, Trends water and nutrients and because of their rapid growth rates they can quickly smother native vegetation. • Rabbit numbers: a DECLINE since Similarly to weeds, many introduced animals compete introduction of Rabbit Haemorrhagic with and predate on native animals and impact on Disease (RHD, also known as calicivirus) native vegetation. They have high reproductive rates although the extent of the decline varies and can tolerate a wide range of habitats. As a result across the State. they often establish populations very quickly. •Fox numbers: DOWN in high priority Weeds can provide shelter for pest animals, conservation areas due to large-scale although they can provide food for or become habitat baiting programs; STILL A PROBLEM in for native animals. Blackberry, for example, is an ideal other parts of the State. habitat for the threatened Southern Brown Bandicoot. This illustrates the complexity of issues associated •Feral camel and deer numbers: UP. with pest control and highlights the need for control •Feral goat numbers: DECLINING across measures to have considered specific conservation Weed affected land – Mount Lofty Ranges the State. outcomes to be undertaken over time and to be Photo: Kym Nicolson •Feral pig numbers: UNKNOWN. -

Resistance of Castanea Clones to Phytophthora Cinnamomi: Testing and Genetic Control
Miranda-Fontaina et. al.·Silvae Genetica (2007) 56-1, 11-21 Resistance of Castanea Clones to Phytophthora Cinnamomi: Testing and Genetic Control By M. E. MIRANDA-FONTAÍÑA1), J. FERNÁNDEZ-LÓPEZ1), A. M. VETTRAINO2) and A. VANNINI2) (Received 23th May 2005) Summary Chestnut hybrid clone forest reproduction material for The resistance of chestnut clones to Phytophthora cin- commercial use in Spain must be approved as belonging namomi was evaluated by a soil inoculation experiment to qualified or controlled categories, and its value must under controlled environmental conditions, as well as by be demonstrated according to the requirements outlined excised and intact stem tests. One-year-old plants of in Directive 1999/105/EC (EUROPEAN COUNCIL, 1999) and fifty different clones were inoculated with two isolates of RD 289/2003 (BOLETIN OFICIAL DEL ESTADO, 2003). For Phytophthora cinnamomi and evaluated fourteen weeks successful wood production in Atlantic areas, stem qual- after inoculation. There were significant differences ity and growth as well as a certain level of resistance to among clones for all root and collar rot variables. There Phytophthora sp. are used as selection criteria. While were significant differences for isolates of P. cinnamomi the first two traits can be assessed in field trials, evalu- but only for the collar rot variables. A total of 84% of ation of resistance in terms of survival is not straightfor- plants grown in infested soil showed symptoms of root rot but only 50% of the plants with root rot, showed also ward, as it is difficult to differentiate the different fac- had collar rot. The roots of resistant clones were able to tors related to survival in the field. -

Frank Meyer, Isabel Shipley Cunningham Agricultural Explorer
Frank Meyer, Isabel Shipley Cunningham Agricultural Explorer For 60 years the work of Frank N. Meyer has 2,500 pages of his letters tell of his journeys remained a neglected segment of America’s and the plants he collected, and the USDA heritage. Now, as people are becoming con- Inventory of Seeds and Plants Imported con- cerned about feeding the world’s growing tains descriptions of his introductions. population and about the loss of genetic di- Until recently little was known about the versity of crops, Meyer’s accomplishments first 25 years of Meyer’s life, when he lived have a special relevance. Entering China in in Amsterdam and was called Frans Meijer. 1905, near the dawn of the single era when Dutch sources reveal that he was bom into a explorers could travel freely there, he be- loving family in 1875. Frans was a quiet boy, came the first plant hunter to represent a who enjoyed taking long walks, reading government and to search primarily for eco- about distant lands, and working in his fami- nomically useful plants rather than orna- ly’s small garden. By the time he had mentals. No one before him had spent 10 fimshed elementary school, he knew that ’ years crossing the mountains, deserts, farms, wanted to be a world traveler who studied he ’,if and forests of Asia in search of fruits, nuts, plants; however, his parents could not afford vegetables, grains, and fodder crops; no one to give him further education. When he was has done so since. 14 years old, he found work as a gardener’s During four plant-hunting expeditions to helper at the Amsterdam Botanical Garden. -

Castanea Mollissima Blume): the Roots of Nut Tree Domestication
ORIGINAL RESEARCH published: 25 June 2018 doi: 10.3389/fpls.2018.00810 Signatures of Selection in the Genomes of Chinese Chestnut (Castanea mollissima Blume): The Roots of Nut Tree Domestication Nicholas R. LaBonte 1*, Peng Zhao 2 and Keith Woeste 3 1 Department of Crop Sciences, University of Illinois Urbana-Champaign, Urbana, IL, United States, 2 Key Laboratory of Resource Biology and Biotechnology in Western China, Ministry of Education, College of Life Sciences, Northwest University, Xi’an, China, 3 Hardwood Tree Improvement and Regeneration Center, Northern Research Station, USDA Forest Service, West Lafayette, IN, United States Chestnuts (Castanea) are major nut crops in East Asia and southern Europe, and are unique among temperate nut crops in that the harvested seeds are starchy rather than oily. Chestnut species have been cultivated for three millennia or more in China, so it is likely that artificial selection has affected the genome of orchard-grown chestnuts. The genetics of Chinese chestnut (Castanea mollissima Blume) domestication are also of Edited by: interest to breeders of hybrid American chestnut, especially if the low-growing, branching S. Hong Lee, habit of Chinese chestnut, an impediment to American chestnut restoration, is partly University of South Australia, Australia the result of artificial selection. We resequenced genomes of wild and orchard-derived Reviewed by: Guo-Bo Chen, Chinese chestnuts and identified selective sweeps based on pooled whole-genome SNP Zhejiang Provincial People’s Hospital, datasets. We present candidate gene loci for chestnut domestication and discuss the China potential phenotypic effects of candidate loci, some of which may be useful genes for Chaeyoung Lee, Soongsil University, South Korea chestnut improvement in Asia and North America. -
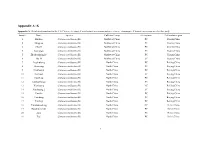
Appendix A~K
Appendix A~K Appendix A. Detailed information for the 146 Chinese chestnut (C.mollissima) accessions and nine chinese chinquapin (C.henryi) accessions used in this study Sample Name Species Cultivars Group Germplasm Cultivation region 1 Huijian Castanea mollissima Bl. Northwest China PC Shanxi,China 2 Mingjian Castanea mollissima Bl. Northwest China PC Shanxi,China 3 Chunli Castanea mollissima Bl. Northwest China PC Shanxi,China 4 Zuohongli Castanea mollissima Bl. Northwest China PC Shanxi,China 5 Zhenbashuangjie Castanea mollissima Bl. Northwest China PC Shanxi,China 6 zha 18 Castanea mollissima Bl. Northwest China LC Shanxi,China 7 Jingshuhong Castanea mollissima Bl. North China PC Beijing,China 8 Huaixiang Castanea mollissima Bl. North China PC Beijing,China 9 Huaihuang Castanea mollissima Bl. North China PC Beijing,China 10 Huzhaoli Castanea mollissima Bl. North China LC Beijing,China 11 Huaifeng Castanea mollissima Bl. North China PC Beijing,China 12 Yanshanhongli Castanea mollissima Bl. North China PC Beijing,China 13 Xiazhuang Castanea mollissima Bl. North China LC Beijing,China 14 Xinzhuang 2 Castanea mollissima Bl. North China LC Beijing,China 15 Huaijiu Castanea mollissima Bl. North China PC Beijing,China 16 Yanchang Castanea mollissima Bl. North China PC Beijing,China 17 Yanfeng Castanea mollissima Bl. North China PC Beijing,China 18 Yanshanzaofeng Castanea mollissima Bl. North China NC Hebei,China 19 Donglimingzhu Castanea mollissima Bl. North China PC Hebei,China 20 Zipo Castanea mollissima Bl. North China PC Hebei,China 21 Tasi Castanea mollissima Bl. North China PC Hebei,China 1 22 Zundali Castanea mollissima Bl. North China PC Hebei,China 23 Duanzhiyabian Castanea mollissima Bl. -

Castanea Mollissima
Castanea mollissima As the American chestnut struggles with disease, the blight- resistant Chinese chestnut is quickly gaining popularity. The sweet-tasting nuts are often roasted for holiday eating and have been made famous in turkey stuffing recipes across the country. But this is more than a nut tree. The shade of its spreading canopy is dense, providing relief in the hot, dry climates the Chinese chestnut does well in. Hardiness Zones: The chinese chestnut can be expected to grow in Hardiness Zones 4-8. Tree Type: This is a nut-producing tree, yielding nuts for human and wildlife consumption. Mature Size: The Chinese chestnut grows to a height of 40-60' and a spread of 40-60' at maturity. Growth Rate: This tree grows at a slow to medium rate, with height increases of anywhere from less than 12" to 24" per year. Sun Preference: Full sun is the ideal condition for this tree, meaning it should get at least six hours of direct, unfiltered sunlight each day. Soil Preference: The Chinese chestnut grows in acidic, loamy, moist, sandy, well-drained, and clay soils. It is drought-tolerant. Attributes This tree: Should be planted in pairs or groups to ensure pollination. 1 Yields a ripened nut crop mid/late September through October. A prickly 2-3 /2" seed husk en- closes 1-4 nuts. The nuts are large, meaty, crisp, and sweet, although less sweet than American chestnuts. Begins to bear nuts in 4-5 years if grown from seed. Provides dense shade with a handsome, spreading canopy. Has wood that is very durable and resistant to rot. -

Presidio Phytophthora Management Recommendations
2016 Presidio Phytophthora Management Recommendations Laura Sims Presidio Phytophthora Management Recommendations (modified) Author: Laura Sims Other Contributing Authors: Christa Conforti, Tom Gordon, Nina Larssen, and Meghan Steinharter Photograph Credits: Laura Sims, Janet Klein, Richard Cobb, Everett Hansen, Thomas Jung, Thomas Cech, and Amelie Rak Editors and Additional Contributors: Christa Conforti, Alison Forrestel, Alisa Shor, Lew Stringer, Sharon Farrell, Teri Thomas, John Doyle, and Kara Mirmelstein Acknowledgements: Thanks first to Matteo Garbelotto and the University of California, Berkeley Forest Pathology and Mycology Lab for providing a ‘forest pathology home’. Many thanks to the members of the Phytophthora huddle group for useful suggestions and feedback. Many thanks to the members of the Working Group for Phytophthoras in Native Habitats for insight into the issues of Phytophthora. Many thanks to Jennifer Parke, Ted Swiecki, Kathy Kosta, Cheryl Blomquist, Susan Frankel, and M. Garbelotto for guidance. I would like to acknowledge the BMP documents on Phytophthora that proceeded this one: the Nursery Industry Best Management Practices for Phytophthora ramorum to prevent the introduction or establishment in California nursery operations, and The Safe Procurement and Production Manual. 1 Title Page: Authors and Acknowledgements Table of Contents Page Title Page 1 Table of Contents 2 Executive Summary 5 Introduction to the Phytophthora Issue 7 Phytophthora Issues Around the World 7 Phytophthora Issues in California 11 Phytophthora -

A Bibliography of Mycology and Plant Pathology in Ireland, 1976 to 2000
Glasra 4: 119 – 188 (2008) A bibliography of mycology and plant pathology in Ireland, 1976 to 2000 A. MANGAN 24 Treesdale, Blackrock, Co. Dublin ABSTRACT This bibliography includes medical and veterinary mycology, and plant pathology relating to bacteriology and virology. It is based on published work from Ireland, between 1976 and 2000 inclusive, and comprises 859 references. An index of topics is included. There is also a list of 193 theses for which Master or Doctorate degrees were awarded. INTRODUCTION In his publication, Mycology and Plant Pathology in Ireland, Muskett (1976) gave an account of the history and development of mycology and plant pathology in Ireland since the first traceable mention of Irish fungi in the scientific sense in 1726. His work included a bibliography containing 1,355 titles, in chronological order, covering the period from 1726 to 1975. The bibliography presented here covers the years 1976 to 2000. The basis of the present work was to search for work on mycology and plant pathology published by authors with an Irish address. Where work relating to Ireland, which was published from an address abroad, was noted, it is included but it was not possible to make a full search for such work. The principal sources for this work were: The Review of Plant Pathology and the Review of Medical and Veterinary Mycology, both published by CAB International. The following journals were also checked for relevant material: Irish Naturalists’ Journal Proceedings of the Royal Irish Academy Proceedings of meetings published by the Royal Irish Academy Scientific Proceedings of the Royal Dublin Society Journal of Life Sciences of the Royal Dublin Society Irish Journal of Agricultural Research later named the Irish Journal of Agricultural and Food Research Mycologist Field Mycology Various reports of meetings, institutes and societies exist, but are not included as they are not readily available in libraries. -

Chestnut Growers Urged to Implement Quarantine for Chestnut Gall Wasp
Vol. 18 No. 4 Published by Chestnut Growers of America, Inc. Fall 2017 Chestnut Growers Urged to Implement Quarantine for Chestnut Gall Wasp By Michelle Warmund, Ph.D., University of Missouri Center for Agroforestry; Tom Green, Ph.D., Professor Emeritus, Western Illinois University; Tom Wahl, Red Fern Farms; Kathy Dice, Red Fern Farms; and Jim Dallmeyer, Thistle Creek Orchard he chestnut gall wasp, Dryocosumus 40 days and the larvae remain dormant Indeed, this pest was first introduced to Tkuriphilus Yasumatsu, is a tiny, gnat- until the following spring, when galls are the US on scion wood. Dispersal by flight sized, non-stinging insect that causes formed. With bud break, larvae induce is eclipsed by human transport. A serious galls in chestnut trees. These galls retard gall formation on developing plant tissues. source of propagation comes from home plant growth and flowering and can kill Larvae feed on the inner gall tissue for 20 owners who plant chestnuts in their yards branches. Severe infestations can kill trees. to 30 days before pupating. Adult wasps and hunters who plant them in woods to After the adult insects emerge, the dried, emerge from the galls in late May and attract deer. While commercial orchards blackened galls become woody and can early June. Beyond the gall clusters of dead may be fairly far apart, these alternate persist on older limbs for several years. leaves form. Called flags, these are easily growers provide additional “stepping Older, slower growing trees are more visible, making location of galls quickly stones” for the spread of the CGW. vulnerable. -

CHESTNUT CULTURE in CALIFORNIA UC Dept
CHESTNUT CULTURE in CALIFORNIA UC Dept. of Ag & Natl. Resources Publication # 8010 - By Paul Vossen The chestnut is a delicious nut produced on large magnificent trees on millions of acres of native habitat in the Northern Hemisphere, particularly China, Korea, Japan, and Southern Europe. The entire Eastern half of the USA was once covered with native chestnut trees until a blight fungus introduced from Asia destroyed them in the early 1900’s. The fleshy nut is sweet with a starchy texture and a low fat content resembling a cereal grain. The nuts are eaten as traditional foods in much of Asia and Europe where they are consumed fresh, cooked, candied, and as a source of flour for pastries. The chestnut tree (Castanea sp.) is in the same family as the beeches and oaks (Fagaceae). The formidable, spiny chestnut burr is the equivalent to the cap on an acorn. Chestnuts belong to the Genus: Castanea, with four main economic species: C. dentata (North American), C. mollissima (Chinese), C. sativa (European), and C. crenata (Japanese). It is not related to the horse chestnut (Aesculus sp.). The tree has a gray bark and is deciduous with leaves 5-7 inches long, sharply serrated, oblong-lanceolate, and pinnately veined. Domestication of the chestnut is still progressing with much of the world’s production collected from natural stands. SPECIES Four species of chestnut are grown in North America. They exist as pure species or, more commonly, as hybrids of the various species because they readily cross with one another. In many cases, it is difficult to distinguish species and almost impossible to determine the parentage of the hybrids visually.