How a Flower Becomes a Chestnut: Morphological Development of Chinese Chestnuts (Castanea Mollisima)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CHESTNUT (CASTANEA Spp.) CULTIVAR EVALUATION for COMMERCIAL CHESTNUT PRODUCTION
CHESTNUT (CASTANEA spp.) CULTIVAR EVALUATION FOR COMMERCIAL CHESTNUT PRODUCTION IN HAMILTON COUNTY, TENNESSEE By Ana Maria Metaxas Approved: James Hill Craddock Jennifer Boyd Professor of Biological Sciences Assistant Professor of Biological and Environmental Sciences (Director of Thesis) (Committee Member) Gregory Reighard Jeffery Elwell Professor of Horticulture Dean, College of Arts and Sciences (Committee Member) A. Jerald Ainsworth Dean of the Graduate School CHESTNUT (CASTANEA spp.) CULTIVAR EVALUATION FOR COMMERCIAL CHESTNUT PRODUCTION IN HAMILTON COUNTY, TENNESSEE by Ana Maria Metaxas A Thesis Submitted to the Faculty of the University of Tennessee at Chattanooga in Partial Fulfillment of the Requirements for the Degree of Master of Science in Environmental Science May 2013 ii ABSTRACT Chestnut cultivars were evaluated for their commercial applicability under the environmental conditions in Hamilton County, TN at 35°13ꞌ 45ꞌꞌ N 85° 00ꞌ 03.97ꞌꞌ W elevation 230 meters. In 2003 and 2004, 534 trees were planted, representing 64 different cultivars, varieties, and species. Twenty trees from each of 20 different cultivars were planted as five-tree plots in a randomized complete block design in four blocks of 100 trees each, amounting to 400 trees. The remaining 44 chestnut cultivars, varieties, and species served as a germplasm collection. These were planted in guard rows surrounding the four blocks in completely randomized, single-tree plots. In the analysis, we investigated our collection predominantly with the aim to: 1) discover the degree of acclimation of grower- recommended cultivars to southeastern Tennessee climatic conditions and 2) ascertain the cultivars’ ability to survive in the area with Cryphonectria parasitica and other chestnut diseases and pests present. -

An Abstract of the Thesis Of
AN ABSTRACT OF THE THESIS OF Annie M. Chozinski for the degree of Master of Science in Horticulture presented on November 23. 1994. Title: The Evaluation of Cold Hardiness in Corvlus. Abstract approved: Shawn A. Mehlenbacher Anthesis of both staminate and pistillate flowers of Cory/us occurs in midwinter. To insure adequate pollination and nut set, these flowers must attain a sufficient hardiness level to withstand low temperatures. This study estimated cold hardiness of Cory/us cultivars and species using laboratory freezing of shoots without artificial hardening. In December, January and February of 1991-92 and 1992-93, one-year stems were collected 0 0 and frozen at regular intervals from -10 C to -38 C/ and visual browning assessed survival approximately 10 days after freezing. Elongated catkins were clipped prior to freezing. Percent flower bud survival was calculated and plotted against temperature. Linear regression generated an equation relating percent bud survival to temperature. From this equation, estimates of the LT^ (lethal temperature for 50% of the buds) was calculated for catkins, female inflorescences, and vegetative buds. C. avellana L. catkins, on average, were less hardy in both December and January than female inflorescences and vegetative buds. Maximum hardiness was reached in December and nearly all had elongated prior to the February freeze. Cultivars with the most hardy catkins were 'Morell', 'Brixnut', 'Creswell', 'Gem', 'Giresun OSU 54.080', 'Hall's Giant', 'Riccia di Talanico', 'Montebello' and 'Rode Zeller'. Maximum hardiness was observed for both vegetative and pistillate buds in January and was followed by a marked loss of hardiness in February. -

Species Profile for California Live Oak (Quercus Agrifolia)
California Phenology Project: species profile for California Live Oak (Quercus agrifolia) CPP site(s) where this species is monitored: Redwood Regional Park, Roberts Regional Recreation Area What does this species look like? This large evergreen tree has a dark grey, stout, short trunk and wide spreading branches. The leathery leaves are shiny on the upper surface and dull on the lower surface, which is covered with fuzzy hairs. The leaf margins are spiny and holly-like. The individuals are monoecious; each tree bears both male and female flowers but the male flowers produce only anthers and the female flowers produce only pistils. The yellow-green male flowers are clustered in elongated, drooping catkins that are 4-10 cm long, and the female flowers are clustered in reddish green spikes. When monitoring this species, use the USA-NPN broadleaf evergreen (with pollen) trees and shrubs Photo credit: randomtruth (Flickr) datasheet. Species facts! • The CPP four letter code for this species is QUAG. • This oak is very fire resistant. Adaptations to fire include evergreen leaves, thick bark, and the ability to sprout post-fire from the roots, trunk, and upper crown. • Individuals can live up to 250 years. • Susceptible to Sudden Oak Death disease. • Wind pollinated. • Each acorn takes a full year to develop from a pollinated flower. Photo credit: randomtruth (Flickr) Where is this species found? • In valleys, slopes, mixed-evergreen forest, and woodlands at elevations less than 1500 meters. • Endemic to California; found in North Coast Ranges, Central Western California, and SW California. • Occurs on soils ranging from silts and clays to weathered granite. -

Salix Caprea (Goat Willow, Great Sallow, Pussy Willow) Goat Willow Is a Small Multi Stemmed Deciduous Tree Native to Europe and Western Asia
Salix caprea (Goat Willow, Great Sallow, Pussy Willow) Goat willow is a small multi stemmed deciduous tree native to Europe and western Asia. It is growing a silky male flower called catkins in early spring Female and male flower grows in a different tree.The leaves are dark green and hairy underneath. Mainly the weeping cultivate is used in gardens. It likes sun and well-drained soil, and benefits from a severe pruning every 2 or 3 years. Grow it where late winter and early spring interest are needed in the garden. Landscape Information French Name: Saule marsault Pronounciation: SAL-iks Plant Type: Tree Origin: Europe and western Asia. Heat Zones: 5, 6, 7, 8, 9 Hardiness Zones: 5, 6, 7, 8, 9 Uses: Screen, Hedge, Specimen, Container, Windbreak, Cut Flowers / Arrangements Size/Shape Growth Rate: Fast Tree Shape: Upright, Weeping Canopy Symmetry: Irregular Canopy Density: Medium Canopy Texture: Medium Height at Maturity: 5 to 8 m Spread at Maturity: 3 to 5 meters Time to Ultimate Height: 10 to 20 Years Plant Image Salix caprea (Goat Willow, Great Sallow, Pussy Willow) Botanical Description Foliage Leaf Arrangement: Alternate Leaf Venation: Pinnate Leaf Persistance: Deciduous Leaf Type: Odd Pinnately compund Leaf Blade: 5 - 10 cm Leaf Shape: Ovate Leaf Margins: Entire, Dentate Leaf Textures: Glossy, Medium Leaf Scent: No Fragance Color(growing season): Green Color(changing season): Green Flower Image Flower Flower Showiness: True Flower Size Range: 1.5 - 3 Flower Type: Catkin Flower Sexuality: Diecious (Monosexual) Flower Scent: No Fragance -

Frank Meyer, Isabel Shipley Cunningham Agricultural Explorer
Frank Meyer, Isabel Shipley Cunningham Agricultural Explorer For 60 years the work of Frank N. Meyer has 2,500 pages of his letters tell of his journeys remained a neglected segment of America’s and the plants he collected, and the USDA heritage. Now, as people are becoming con- Inventory of Seeds and Plants Imported con- cerned about feeding the world’s growing tains descriptions of his introductions. population and about the loss of genetic di- Until recently little was known about the versity of crops, Meyer’s accomplishments first 25 years of Meyer’s life, when he lived have a special relevance. Entering China in in Amsterdam and was called Frans Meijer. 1905, near the dawn of the single era when Dutch sources reveal that he was bom into a explorers could travel freely there, he be- loving family in 1875. Frans was a quiet boy, came the first plant hunter to represent a who enjoyed taking long walks, reading government and to search primarily for eco- about distant lands, and working in his fami- nomically useful plants rather than orna- ly’s small garden. By the time he had mentals. No one before him had spent 10 fimshed elementary school, he knew that ’ years crossing the mountains, deserts, farms, wanted to be a world traveler who studied he ’,if and forests of Asia in search of fruits, nuts, plants; however, his parents could not afford vegetables, grains, and fodder crops; no one to give him further education. When he was has done so since. 14 years old, he found work as a gardener’s During four plant-hunting expeditions to helper at the Amsterdam Botanical Garden. -

Castanea Mollissima Blume): the Roots of Nut Tree Domestication
ORIGINAL RESEARCH published: 25 June 2018 doi: 10.3389/fpls.2018.00810 Signatures of Selection in the Genomes of Chinese Chestnut (Castanea mollissima Blume): The Roots of Nut Tree Domestication Nicholas R. LaBonte 1*, Peng Zhao 2 and Keith Woeste 3 1 Department of Crop Sciences, University of Illinois Urbana-Champaign, Urbana, IL, United States, 2 Key Laboratory of Resource Biology and Biotechnology in Western China, Ministry of Education, College of Life Sciences, Northwest University, Xi’an, China, 3 Hardwood Tree Improvement and Regeneration Center, Northern Research Station, USDA Forest Service, West Lafayette, IN, United States Chestnuts (Castanea) are major nut crops in East Asia and southern Europe, and are unique among temperate nut crops in that the harvested seeds are starchy rather than oily. Chestnut species have been cultivated for three millennia or more in China, so it is likely that artificial selection has affected the genome of orchard-grown chestnuts. The genetics of Chinese chestnut (Castanea mollissima Blume) domestication are also of Edited by: interest to breeders of hybrid American chestnut, especially if the low-growing, branching S. Hong Lee, habit of Chinese chestnut, an impediment to American chestnut restoration, is partly University of South Australia, Australia the result of artificial selection. We resequenced genomes of wild and orchard-derived Reviewed by: Guo-Bo Chen, Chinese chestnuts and identified selective sweeps based on pooled whole-genome SNP Zhejiang Provincial People’s Hospital, datasets. We present candidate gene loci for chestnut domestication and discuss the China potential phenotypic effects of candidate loci, some of which may be useful genes for Chaeyoung Lee, Soongsil University, South Korea chestnut improvement in Asia and North America. -
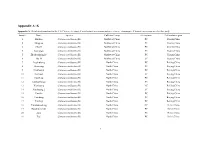
Appendix A~K
Appendix A~K Appendix A. Detailed information for the 146 Chinese chestnut (C.mollissima) accessions and nine chinese chinquapin (C.henryi) accessions used in this study Sample Name Species Cultivars Group Germplasm Cultivation region 1 Huijian Castanea mollissima Bl. Northwest China PC Shanxi,China 2 Mingjian Castanea mollissima Bl. Northwest China PC Shanxi,China 3 Chunli Castanea mollissima Bl. Northwest China PC Shanxi,China 4 Zuohongli Castanea mollissima Bl. Northwest China PC Shanxi,China 5 Zhenbashuangjie Castanea mollissima Bl. Northwest China PC Shanxi,China 6 zha 18 Castanea mollissima Bl. Northwest China LC Shanxi,China 7 Jingshuhong Castanea mollissima Bl. North China PC Beijing,China 8 Huaixiang Castanea mollissima Bl. North China PC Beijing,China 9 Huaihuang Castanea mollissima Bl. North China PC Beijing,China 10 Huzhaoli Castanea mollissima Bl. North China LC Beijing,China 11 Huaifeng Castanea mollissima Bl. North China PC Beijing,China 12 Yanshanhongli Castanea mollissima Bl. North China PC Beijing,China 13 Xiazhuang Castanea mollissima Bl. North China LC Beijing,China 14 Xinzhuang 2 Castanea mollissima Bl. North China LC Beijing,China 15 Huaijiu Castanea mollissima Bl. North China PC Beijing,China 16 Yanchang Castanea mollissima Bl. North China PC Beijing,China 17 Yanfeng Castanea mollissima Bl. North China PC Beijing,China 18 Yanshanzaofeng Castanea mollissima Bl. North China NC Hebei,China 19 Donglimingzhu Castanea mollissima Bl. North China PC Hebei,China 20 Zipo Castanea mollissima Bl. North China PC Hebei,China 21 Tasi Castanea mollissima Bl. North China PC Hebei,China 1 22 Zundali Castanea mollissima Bl. North China PC Hebei,China 23 Duanzhiyabian Castanea mollissima Bl. -

Castanea Mollissima
Castanea mollissima As the American chestnut struggles with disease, the blight- resistant Chinese chestnut is quickly gaining popularity. The sweet-tasting nuts are often roasted for holiday eating and have been made famous in turkey stuffing recipes across the country. But this is more than a nut tree. The shade of its spreading canopy is dense, providing relief in the hot, dry climates the Chinese chestnut does well in. Hardiness Zones: The chinese chestnut can be expected to grow in Hardiness Zones 4-8. Tree Type: This is a nut-producing tree, yielding nuts for human and wildlife consumption. Mature Size: The Chinese chestnut grows to a height of 40-60' and a spread of 40-60' at maturity. Growth Rate: This tree grows at a slow to medium rate, with height increases of anywhere from less than 12" to 24" per year. Sun Preference: Full sun is the ideal condition for this tree, meaning it should get at least six hours of direct, unfiltered sunlight each day. Soil Preference: The Chinese chestnut grows in acidic, loamy, moist, sandy, well-drained, and clay soils. It is drought-tolerant. Attributes This tree: Should be planted in pairs or groups to ensure pollination. 1 Yields a ripened nut crop mid/late September through October. A prickly 2-3 /2" seed husk en- closes 1-4 nuts. The nuts are large, meaty, crisp, and sweet, although less sweet than American chestnuts. Begins to bear nuts in 4-5 years if grown from seed. Provides dense shade with a handsome, spreading canopy. Has wood that is very durable and resistant to rot. -

20-053 — Provisional Recommendations And
Factsheet #20-053 | AGDEX 240 | AUGUST 2020 Provisional Recommendations and Descriptions of Hazelnut Varieties in Ontario T. Leuty INTRODUCTION potential market uses and yield and sensitivity to The Ontario hazelnut industry is currently in a state winter injury (hardiness zone). of early commercial development. Hazelnut cultivars are being evaluated as potential crop cultivars and Hazelnuts are self-incompatible. This means that pollen source cultivars, based on their ability to a hazelnut plant will not pollenize its own female tolerate minimum winter temperatures, spring frost flowers or pollenize female flowers of other and manage native diseases, mainly Eastern filbert hazelnut plants within the same variety. Hazelnut blight (EFB) and bacterial blight. Maturing orchards varieties must cross-pollenize with other compatible have begun to produce an annual hazelnut crop. varieties in order to set and grow a nut crop. It is Kernels are being evaluated for quality attributes recommended that growers provide three to five and for various market uses including fresh, different compatible pollen source varieties for each confectionary and other value-added products. crop cultivar to ensure successful pollen transfer and optimize crop yield. The timing of pollen Table 1 provides provisional hazelnut variety release is also a factor. Male catkin flowers should recommendations for Ontario with revised release pollen when the female crop flowers are tolerance/susceptibility to Eastern filbert blight, open and receptive. Table 1. Provisional -

Chestnut Growers Urged to Implement Quarantine for Chestnut Gall Wasp
Vol. 18 No. 4 Published by Chestnut Growers of America, Inc. Fall 2017 Chestnut Growers Urged to Implement Quarantine for Chestnut Gall Wasp By Michelle Warmund, Ph.D., University of Missouri Center for Agroforestry; Tom Green, Ph.D., Professor Emeritus, Western Illinois University; Tom Wahl, Red Fern Farms; Kathy Dice, Red Fern Farms; and Jim Dallmeyer, Thistle Creek Orchard he chestnut gall wasp, Dryocosumus 40 days and the larvae remain dormant Indeed, this pest was first introduced to Tkuriphilus Yasumatsu, is a tiny, gnat- until the following spring, when galls are the US on scion wood. Dispersal by flight sized, non-stinging insect that causes formed. With bud break, larvae induce is eclipsed by human transport. A serious galls in chestnut trees. These galls retard gall formation on developing plant tissues. source of propagation comes from home plant growth and flowering and can kill Larvae feed on the inner gall tissue for 20 owners who plant chestnuts in their yards branches. Severe infestations can kill trees. to 30 days before pupating. Adult wasps and hunters who plant them in woods to After the adult insects emerge, the dried, emerge from the galls in late May and attract deer. While commercial orchards blackened galls become woody and can early June. Beyond the gall clusters of dead may be fairly far apart, these alternate persist on older limbs for several years. leaves form. Called flags, these are easily growers provide additional “stepping Older, slower growing trees are more visible, making location of galls quickly stones” for the spread of the CGW. vulnerable. -

CHESTNUT CULTURE in CALIFORNIA UC Dept
CHESTNUT CULTURE in CALIFORNIA UC Dept. of Ag & Natl. Resources Publication # 8010 - By Paul Vossen The chestnut is a delicious nut produced on large magnificent trees on millions of acres of native habitat in the Northern Hemisphere, particularly China, Korea, Japan, and Southern Europe. The entire Eastern half of the USA was once covered with native chestnut trees until a blight fungus introduced from Asia destroyed them in the early 1900’s. The fleshy nut is sweet with a starchy texture and a low fat content resembling a cereal grain. The nuts are eaten as traditional foods in much of Asia and Europe where they are consumed fresh, cooked, candied, and as a source of flour for pastries. The chestnut tree (Castanea sp.) is in the same family as the beeches and oaks (Fagaceae). The formidable, spiny chestnut burr is the equivalent to the cap on an acorn. Chestnuts belong to the Genus: Castanea, with four main economic species: C. dentata (North American), C. mollissima (Chinese), C. sativa (European), and C. crenata (Japanese). It is not related to the horse chestnut (Aesculus sp.). The tree has a gray bark and is deciduous with leaves 5-7 inches long, sharply serrated, oblong-lanceolate, and pinnately veined. Domestication of the chestnut is still progressing with much of the world’s production collected from natural stands. SPECIES Four species of chestnut are grown in North America. They exist as pure species or, more commonly, as hybrids of the various species because they readily cross with one another. In many cases, it is difficult to distinguish species and almost impossible to determine the parentage of the hybrids visually. -

Descriptors for Hazelnut (Corylus Avellana L.)
Descriptors for Hazelnut(Corylus avellana L.) List of Descriptors Allium (E, S) 2001 Pearl millet (E/F) 1993 Almond (revised)* (E) 1985 Pepino (E) 2004 Apple* (E) 1982 Phaseolus acutifolius (E) 1985 Apricot* (E) 1984 Phaseolus coccineus* (E) 1983 Avocado (E/S) 1995 Phaseolus lunatus (P) 2001 Bambara groundnut (E, F) 2000 Phaseolus vulgaris* (E, P) 1982 Banana (E, S, F) 1996 Pigeonpea (E) 1993 Barley (E) 1994 Pineapple (E) 1991 Beta (E) 1991 Pistachio (A, R, E, F) 1997 Black pepper (E/S) 1995 Pistacia (excluding Pistacia vera) (E) 1998 Brassica and Raphanus (E) 1990 Plum* (E) 1985 Brassica campestris L. (E) 1987 Potato variety* (E) 1985 Buckwheat (E) 1994 Quinua* (E) 1981 Cañahua (S) 2005 Rambutan 2003 Capsicum (E/S) 1995 Rice* (E) 2007 Cardamom (E) 1994 Rocket (E, I) 1999 Carrot (E, S, F) 1998 Rye and Triticale* (E) 1985 Cashew* (E) 1986 Safflower* (E) 1983 Cherry* (E) 1985 Sesame (E) 2004 Chickpea (E) 1993 Setaria italica and S. pumilia (E) 1985 Citrus (E, F, S) 1999 Shea tree (E) 2006 Coconut (E) 1995 Sorghum (E/F) 1993 Coffee (E, S, F) 1996 Soyabean* (E/C) 1984 Cotton (revised)* (E) 1985 Strawberry (E) 1986 Cowpea (E, P)* 1983 Sunflower* (E) 1985 Cultivated potato* (E) 1977 Sweet potato (E/S/F) 1991 Date Palm (F) 2005 Taro (E, F, S) 1999 Durian (E) 2007 Tea (E, S, F) 1997 Echinochloa millet* (E) 1983 Tomato (E, S, F) 1996 Eggplant (E/F) 1990 Tropical fruit (revised)* (E) 1980 Faba bean* (E) 1985 Ulluco (S) 2003 Fig (E) 2003 Vigna aconitifolia and V.