Chestnuts Bred for Blight Resistance Depart Nursery with Distinct Fungal Rhizobiomes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CHESTNUT (CASTANEA Spp.) CULTIVAR EVALUATION for COMMERCIAL CHESTNUT PRODUCTION
CHESTNUT (CASTANEA spp.) CULTIVAR EVALUATION FOR COMMERCIAL CHESTNUT PRODUCTION IN HAMILTON COUNTY, TENNESSEE By Ana Maria Metaxas Approved: James Hill Craddock Jennifer Boyd Professor of Biological Sciences Assistant Professor of Biological and Environmental Sciences (Director of Thesis) (Committee Member) Gregory Reighard Jeffery Elwell Professor of Horticulture Dean, College of Arts and Sciences (Committee Member) A. Jerald Ainsworth Dean of the Graduate School CHESTNUT (CASTANEA spp.) CULTIVAR EVALUATION FOR COMMERCIAL CHESTNUT PRODUCTION IN HAMILTON COUNTY, TENNESSEE by Ana Maria Metaxas A Thesis Submitted to the Faculty of the University of Tennessee at Chattanooga in Partial Fulfillment of the Requirements for the Degree of Master of Science in Environmental Science May 2013 ii ABSTRACT Chestnut cultivars were evaluated for their commercial applicability under the environmental conditions in Hamilton County, TN at 35°13ꞌ 45ꞌꞌ N 85° 00ꞌ 03.97ꞌꞌ W elevation 230 meters. In 2003 and 2004, 534 trees were planted, representing 64 different cultivars, varieties, and species. Twenty trees from each of 20 different cultivars were planted as five-tree plots in a randomized complete block design in four blocks of 100 trees each, amounting to 400 trees. The remaining 44 chestnut cultivars, varieties, and species served as a germplasm collection. These were planted in guard rows surrounding the four blocks in completely randomized, single-tree plots. In the analysis, we investigated our collection predominantly with the aim to: 1) discover the degree of acclimation of grower- recommended cultivars to southeastern Tennessee climatic conditions and 2) ascertain the cultivars’ ability to survive in the area with Cryphonectria parasitica and other chestnut diseases and pests present. -

Frank Meyer, Isabel Shipley Cunningham Agricultural Explorer
Frank Meyer, Isabel Shipley Cunningham Agricultural Explorer For 60 years the work of Frank N. Meyer has 2,500 pages of his letters tell of his journeys remained a neglected segment of America’s and the plants he collected, and the USDA heritage. Now, as people are becoming con- Inventory of Seeds and Plants Imported con- cerned about feeding the world’s growing tains descriptions of his introductions. population and about the loss of genetic di- Until recently little was known about the versity of crops, Meyer’s accomplishments first 25 years of Meyer’s life, when he lived have a special relevance. Entering China in in Amsterdam and was called Frans Meijer. 1905, near the dawn of the single era when Dutch sources reveal that he was bom into a explorers could travel freely there, he be- loving family in 1875. Frans was a quiet boy, came the first plant hunter to represent a who enjoyed taking long walks, reading government and to search primarily for eco- about distant lands, and working in his fami- nomically useful plants rather than orna- ly’s small garden. By the time he had mentals. No one before him had spent 10 fimshed elementary school, he knew that ’ years crossing the mountains, deserts, farms, wanted to be a world traveler who studied he ’,if and forests of Asia in search of fruits, nuts, plants; however, his parents could not afford vegetables, grains, and fodder crops; no one to give him further education. When he was has done so since. 14 years old, he found work as a gardener’s During four plant-hunting expeditions to helper at the Amsterdam Botanical Garden. -

Castanea Mollissima Blume): the Roots of Nut Tree Domestication
ORIGINAL RESEARCH published: 25 June 2018 doi: 10.3389/fpls.2018.00810 Signatures of Selection in the Genomes of Chinese Chestnut (Castanea mollissima Blume): The Roots of Nut Tree Domestication Nicholas R. LaBonte 1*, Peng Zhao 2 and Keith Woeste 3 1 Department of Crop Sciences, University of Illinois Urbana-Champaign, Urbana, IL, United States, 2 Key Laboratory of Resource Biology and Biotechnology in Western China, Ministry of Education, College of Life Sciences, Northwest University, Xi’an, China, 3 Hardwood Tree Improvement and Regeneration Center, Northern Research Station, USDA Forest Service, West Lafayette, IN, United States Chestnuts (Castanea) are major nut crops in East Asia and southern Europe, and are unique among temperate nut crops in that the harvested seeds are starchy rather than oily. Chestnut species have been cultivated for three millennia or more in China, so it is likely that artificial selection has affected the genome of orchard-grown chestnuts. The genetics of Chinese chestnut (Castanea mollissima Blume) domestication are also of Edited by: interest to breeders of hybrid American chestnut, especially if the low-growing, branching S. Hong Lee, habit of Chinese chestnut, an impediment to American chestnut restoration, is partly University of South Australia, Australia the result of artificial selection. We resequenced genomes of wild and orchard-derived Reviewed by: Guo-Bo Chen, Chinese chestnuts and identified selective sweeps based on pooled whole-genome SNP Zhejiang Provincial People’s Hospital, datasets. We present candidate gene loci for chestnut domestication and discuss the China potential phenotypic effects of candidate loci, some of which may be useful genes for Chaeyoung Lee, Soongsil University, South Korea chestnut improvement in Asia and North America. -
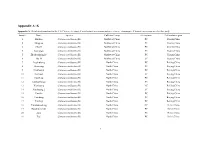
Appendix A~K
Appendix A~K Appendix A. Detailed information for the 146 Chinese chestnut (C.mollissima) accessions and nine chinese chinquapin (C.henryi) accessions used in this study Sample Name Species Cultivars Group Germplasm Cultivation region 1 Huijian Castanea mollissima Bl. Northwest China PC Shanxi,China 2 Mingjian Castanea mollissima Bl. Northwest China PC Shanxi,China 3 Chunli Castanea mollissima Bl. Northwest China PC Shanxi,China 4 Zuohongli Castanea mollissima Bl. Northwest China PC Shanxi,China 5 Zhenbashuangjie Castanea mollissima Bl. Northwest China PC Shanxi,China 6 zha 18 Castanea mollissima Bl. Northwest China LC Shanxi,China 7 Jingshuhong Castanea mollissima Bl. North China PC Beijing,China 8 Huaixiang Castanea mollissima Bl. North China PC Beijing,China 9 Huaihuang Castanea mollissima Bl. North China PC Beijing,China 10 Huzhaoli Castanea mollissima Bl. North China LC Beijing,China 11 Huaifeng Castanea mollissima Bl. North China PC Beijing,China 12 Yanshanhongli Castanea mollissima Bl. North China PC Beijing,China 13 Xiazhuang Castanea mollissima Bl. North China LC Beijing,China 14 Xinzhuang 2 Castanea mollissima Bl. North China LC Beijing,China 15 Huaijiu Castanea mollissima Bl. North China PC Beijing,China 16 Yanchang Castanea mollissima Bl. North China PC Beijing,China 17 Yanfeng Castanea mollissima Bl. North China PC Beijing,China 18 Yanshanzaofeng Castanea mollissima Bl. North China NC Hebei,China 19 Donglimingzhu Castanea mollissima Bl. North China PC Hebei,China 20 Zipo Castanea mollissima Bl. North China PC Hebei,China 21 Tasi Castanea mollissima Bl. North China PC Hebei,China 1 22 Zundali Castanea mollissima Bl. North China PC Hebei,China 23 Duanzhiyabian Castanea mollissima Bl. -

Castanea Mollissima
Castanea mollissima As the American chestnut struggles with disease, the blight- resistant Chinese chestnut is quickly gaining popularity. The sweet-tasting nuts are often roasted for holiday eating and have been made famous in turkey stuffing recipes across the country. But this is more than a nut tree. The shade of its spreading canopy is dense, providing relief in the hot, dry climates the Chinese chestnut does well in. Hardiness Zones: The chinese chestnut can be expected to grow in Hardiness Zones 4-8. Tree Type: This is a nut-producing tree, yielding nuts for human and wildlife consumption. Mature Size: The Chinese chestnut grows to a height of 40-60' and a spread of 40-60' at maturity. Growth Rate: This tree grows at a slow to medium rate, with height increases of anywhere from less than 12" to 24" per year. Sun Preference: Full sun is the ideal condition for this tree, meaning it should get at least six hours of direct, unfiltered sunlight each day. Soil Preference: The Chinese chestnut grows in acidic, loamy, moist, sandy, well-drained, and clay soils. It is drought-tolerant. Attributes This tree: Should be planted in pairs or groups to ensure pollination. 1 Yields a ripened nut crop mid/late September through October. A prickly 2-3 /2" seed husk en- closes 1-4 nuts. The nuts are large, meaty, crisp, and sweet, although less sweet than American chestnuts. Begins to bear nuts in 4-5 years if grown from seed. Provides dense shade with a handsome, spreading canopy. Has wood that is very durable and resistant to rot. -

Chestnut Growers Urged to Implement Quarantine for Chestnut Gall Wasp
Vol. 18 No. 4 Published by Chestnut Growers of America, Inc. Fall 2017 Chestnut Growers Urged to Implement Quarantine for Chestnut Gall Wasp By Michelle Warmund, Ph.D., University of Missouri Center for Agroforestry; Tom Green, Ph.D., Professor Emeritus, Western Illinois University; Tom Wahl, Red Fern Farms; Kathy Dice, Red Fern Farms; and Jim Dallmeyer, Thistle Creek Orchard he chestnut gall wasp, Dryocosumus 40 days and the larvae remain dormant Indeed, this pest was first introduced to Tkuriphilus Yasumatsu, is a tiny, gnat- until the following spring, when galls are the US on scion wood. Dispersal by flight sized, non-stinging insect that causes formed. With bud break, larvae induce is eclipsed by human transport. A serious galls in chestnut trees. These galls retard gall formation on developing plant tissues. source of propagation comes from home plant growth and flowering and can kill Larvae feed on the inner gall tissue for 20 owners who plant chestnuts in their yards branches. Severe infestations can kill trees. to 30 days before pupating. Adult wasps and hunters who plant them in woods to After the adult insects emerge, the dried, emerge from the galls in late May and attract deer. While commercial orchards blackened galls become woody and can early June. Beyond the gall clusters of dead may be fairly far apart, these alternate persist on older limbs for several years. leaves form. Called flags, these are easily growers provide additional “stepping Older, slower growing trees are more visible, making location of galls quickly stones” for the spread of the CGW. vulnerable. -

CHESTNUT CULTURE in CALIFORNIA UC Dept
CHESTNUT CULTURE in CALIFORNIA UC Dept. of Ag & Natl. Resources Publication # 8010 - By Paul Vossen The chestnut is a delicious nut produced on large magnificent trees on millions of acres of native habitat in the Northern Hemisphere, particularly China, Korea, Japan, and Southern Europe. The entire Eastern half of the USA was once covered with native chestnut trees until a blight fungus introduced from Asia destroyed them in the early 1900’s. The fleshy nut is sweet with a starchy texture and a low fat content resembling a cereal grain. The nuts are eaten as traditional foods in much of Asia and Europe where they are consumed fresh, cooked, candied, and as a source of flour for pastries. The chestnut tree (Castanea sp.) is in the same family as the beeches and oaks (Fagaceae). The formidable, spiny chestnut burr is the equivalent to the cap on an acorn. Chestnuts belong to the Genus: Castanea, with four main economic species: C. dentata (North American), C. mollissima (Chinese), C. sativa (European), and C. crenata (Japanese). It is not related to the horse chestnut (Aesculus sp.). The tree has a gray bark and is deciduous with leaves 5-7 inches long, sharply serrated, oblong-lanceolate, and pinnately veined. Domestication of the chestnut is still progressing with much of the world’s production collected from natural stands. SPECIES Four species of chestnut are grown in North America. They exist as pure species or, more commonly, as hybrids of the various species because they readily cross with one another. In many cases, it is difficult to distinguish species and almost impossible to determine the parentage of the hybrids visually. -

The Chestnut Blight Fungus for Studies on Virus/Host and Virus/Virus Interactions: from a Natural to a Model Host
Virology 477 (2015) 164–175 Contents lists available at ScienceDirect Virology journal homepage: www.elsevier.com/locate/yviro The chestnut blight fungus for studies on virus/host and virus/virus interactions: From a natural to a model host Ana Eusebio-Cope a, Liying Sun b, Toru Tanaka a, Sotaro Chiba a, Shin Kasahara c, Nobuhiro Suzuki a,n a Institute of Plant Science and Resources (IPSR), Okayama University, Chuou 2-20-1, Kurashiki, Okayama 710-0046, Japan b College of Plant Protection, Northwest A & F University, Yangling, Shananxi, China c Department of Environmental Sciences, Miyagi University, Sendai 982-215, Japan article info abstract Article history: The chestnut blight fungus, Cryphonectria parasitica, is an important plant pathogenic ascomycete. The Received 16 August 2014 fungus hosts a wide range of viruses and now has been established as a model filamentous fungus for Returned to author for revisions studying virus/host and virus/virus interactions. This is based on the development of methods for 15 September 2014 artificial virus introduction and elimination, host genome manipulability, available host genome Accepted 26 September 2014 sequence with annotations, host mutant strains, and molecular tools. Molecular tools include sub- Available online 4 November 2014 cellular distribution markers, gene expression reporters, and vectors with regulatable promoters that Keywords: have been long available for unicellular organisms, cultured cells, individuals of animals and plants, and Cryphonectria parasitica certain filamentous fungi. A comparison with other filamentous fungi such as Neurospora crassa has been Chestnut blight fungus made to establish clear advantages and disadvantages of C. parasitica as a virus host. In addition, a few dsRNA recent studies on RNA silencing vs. -

Pollen Morphology of Chestnut Cultivars in Southern China by Using Scanning Electron Microscope
Bangladesh J. Bot. 49(1): 39-46, 2020 (March) POLLEN MORPHOLOGY OF CHESTNUT CULTIVARS IN SOUTHERN CHINA BY USING SCANNING ELECTRON MICROSCOPE HUAN XIONG, DE-YI YUAN, YA CHEN, GENHUA NIU AND FENG ZOU* The Key Lab of Non-wood Forest Products of Forestry Ministry , Central South University of Forestry and Technology, Changsha 410004, Hunan, P. R. China Keywords: Pollen morphology, Chestnut, Pollen axis length, Equatorial width, Colpus length Abstract The morphological characteristics of pollen grains of 16 cultivars belonging to two species of the genus Castanea (Castanea mollissima and C. henryi) collected from southern China were observed by using scanning electron microscope (SEM). The length of polar axis (PL), the equatorial diameter (ED), the length of colpus (CL) of grain, P/E ratio (polar axis to equatorial diameter), P/C ratio (polar axis to colpus length), and C/E ratio (colpus length to equatorial diameter) were measured and their variations were compared among studied taxa. The result of this study indicated that chestnut pollen grain was a tricolporate in aperture type, and aperprolate type in equatorial view. The morphological characteristics were mainly represented by PL, ED and CL as revealed by principal components analysis, which accounted for 99.56% of the total variations. The cluster analysis by the UPGMA tree divided the taxa into three groups and showed a great morphological affinity among the 16 cultivars. Introduction Chestnut has been placed in the family of Fagaceae which is monoecious, wind-pollinated and self-incompatible (Mckay 1942, Bounous and Marinoni 2005). In China, this species is economically important for timber and nut production. -

NDP 11 V2 - National Diagnostic Protocol for Cryphonectria Parasitica
NDP 11 V2 - National Diagnostic Protocol for Cryphonectria parasitica National Diagnostic Protocol Chestnut blight Caused by Cryphonectria parasitica NDP 11 V2 NDP 11 V2 - National Diagnostic Protocol for Cryphonectria parasitica © Commonwealth of Australia Ownership of intellectual property rights Unless otherwise noted, copyright (and any other intellectual property rights, if any) in this publication is owned by the Commonwealth of Australia (referred to as the Commonwealth). Creative Commons licence All material in this publication is licensed under a Creative Commons Attribution 3.0 Australia Licence, save for content supplied by third parties, logos and the Commonwealth Coat of Arms. Creative Commons Attribution 3.0 Australia Licence is a standard form licence agreement that allows you to copy, distribute, transmit and adapt this publication provided you attribute the work. A summary of the licence terms is available from http://creativecommons.org/licenses/by/3.0/au/deed.en. The full licence terms are available from https://creativecommons.org/licenses/by/3.0/au/legalcode. This publication (and any material sourced from it) should be attributed as: Subcommittee on Plant Health Diagnostics (2017). National Diagnostic Protocol for Cryphonectria parasitica – NDP11 V2. (Eds. Subcommittee on Plant Health Diagnostics) Authors Cunnington, J, Mohammed, C and Glen, M. Reviewers Pascoe, I and Tan YP, ISBN 978-0- 9945113-6-2. CC BY 3.0. Cataloguing data Subcommittee on Plant Health Diagnostics (2017). National Diagnostic Protocol for Cryphonectria parasitica – NDP11 V2. (Eds. Subcommittee on Plant Health Diagnostics) Authors Cunnington, J, Mohammed, C and Glen, M. Reviewers Pascoe, I and Tan YP, ISBN 978-0-9945113-6-2. -

Restoration of the American Chestnut in New Jersey
U.S. Fish & Wildlife Service Restoration of the American Chestnut in New Jersey The American chestnut (Castanea dentata) is a tree native to New Jersey that once grew from Maine to Mississippi and as far west as Indiana and Tennessee. This tree with wide-spreading branches and a deep broad-rounded crown can live 500-800 years and reach a height of 100 feet and a diameter of more than 10 feet. Once estimated at 4 billion trees, the American chestnut Harvested chestnuts, early 1900's. has almost been extirpated in the last 100 years. The U.S. Fish and Wildlife Service, New Jersey Field Value Office (Service) and its partners, including American Chestnut The American chestnut is valued Cooperators’ Foundation, American for its fruit and lumber. Chestnuts Chestnut Foundation, Monmouth are referred to as the “bread County Parks, Bayside State tree” because their nuts are Prison, Natural Lands Trust, and so high in starch that they can several volunteers, are working to American chestnut leaf (4"-8"). be milled into flour. Chestnuts recover the American chestnut in can be roasted, boiled, dried, or New Jersey. History candied. The nuts that fell to the ground were an important cash Chestnuts have a long history of crop for families in the northeast cultivation and use. The European U.S. and southern Appalachians chestnut (Castanea sativa) formed up until the twentieth century. the basis of a vital economy in Chestnuts were taken into towns the Mediterranean Basin during by wagonload and then shipped Roman times. More recently, by train to major markets in New areas in Southern Europe (such as York, Boston, and Philadelphia. -

Ectomycorrhizal Fungal Community Structure in a Young Orchard of Grafted and Ungrafted Hybrid Chestnut Saplings
Mycorrhiza (2021) 31:189–201 https://doi.org/10.1007/s00572-020-01015-0 ORIGINAL ARTICLE Ectomycorrhizal fungal community structure in a young orchard of grafted and ungrafted hybrid chestnut saplings Serena Santolamazza‑Carbone1,2 · Laura Iglesias‑Bernabé1 · Esteban Sinde‑Stompel3 · Pedro Pablo Gallego1,2 Received: 29 August 2020 / Accepted: 17 December 2020 / Published online: 27 January 2021 © The Author(s) 2021 Abstract Ectomycorrhizal (ECM) fungal community of the European chestnut has been poorly investigated, and mostly by sporocarp sampling. We proposed the study of the ECM fungal community of 2-year-old chestnut hybrids Castanea × coudercii (Castanea sativa × Castanea crenata) using molecular approaches. By using the chestnut hybrid clones 111 and 125, we assessed the impact of grafting on ECM colonization rate, species diversity, and fungal community composition. The clone type did not have an impact on the studied variables; however, grafting signifcantly infuenced ECM colonization rate in clone 111. Species diversity and richness did not vary between the experimental groups. Grafted and ungrafted plants of clone 111 had a diferent ECM fungal species composition. Sequence data from ITS regions of rDNA revealed the presence of 9 orders, 15 families, 19 genera, and 27 species of ECM fungi, most of them generalist, early-stage species. Thirteen new taxa were described in association with chestnuts. The basidiomycetes Agaricales (13 taxa) and Boletales (11 taxa) represented 36% and 31%, of the total sampled ECM fungal taxa, respectively. Scleroderma citrinum, S. areolatum, and S. polyrhizum (Boletales) were found in 86% of the trees and represented 39% of total ECM root tips. The ascomycete Cenococcum geophilum (Mytilinidiales) was found in 80% of the trees but accounted only for 6% of the colonized root tips.