Molecular Genetics Applications: 13.7) Practical Applications of DNA
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Curr.Opin.Chem. Biol., 15, 587-594
Available online at www.sciencedirect.com Bacterial replicases and related polymerases Charles S McHenry Bacterial replicases are complex, tripartite replicative replication that are conserved among all life forms are machines. They contain a polymerase, Pol III, a b2 processivity illustrated in Figure 1. factor and a DnaX complex ATPase that loads b2 onto DNA and chaperones Pol III onto the newly loaded b2. Many Structure and function of a, the catalytic bacteria encode both a full length t and a shorter g form of subunit DnaX by a variety of mechanisms. The polymerase catalytic Like all polymerases, Pol III a contains palm, thumb and subunit of Pol III, a, contains a PHP domain that not only binds fingers domains, in the shape of a cupped right hand 2+ to prototypical e Mg -dependent exonuclease, but also Figure 2. However, apo-enzyme structures of the full 2+ contains a second Zn -dependent proofreading length Thermus aquaticus (Taq) and a truncated version of exonuclease, at least in some bacteria. Replication of the E. coli (Eco) a subunit revealed a big surprise: the palm chromosomes of low GC Gram-positive bacteria require two domain has the basic fold of the X family of DNA Pol IIIs, one of which, DnaE, appears to extend RNA primers a polymerases, which includes the slow, non-processive only short distance before handing the product off to the major Pol bs [1 ,2 ]. replicase, PolC. Other bacteria encode a second Pol III (ImuC) that apparently replaces Pol V, required for induced A ternary complex of a dideoxy-terminated primer-tem- mutagenesis in E. -
Supplementary Data TMAO Microbiome R1
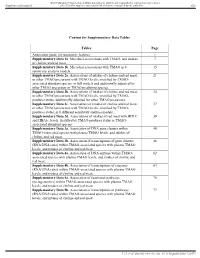
BMJ Publishing Group Limited (BMJ) disclaims all liability and responsibility arising from any reliance Supplemental material placed on this supplemental material which has been supplied by the author(s) Gut Content for Supplementary Data Tables Tables Page Annotation guide for taxonomic features. 1 Supplementary Data 1a. Microbial associations with TMAO, and intakes 7 of choline and red meat. Supplementary Data 1b. Microbial associations with TMAO in 8 15 sensitivity analysis models. Supplementary Data 2a. Associations of intakes of choline and red meat, 21 or other TMAO precursors with TMAO levels, stratified by TMAO- associated abundant species, in full models and additionally adjusted for other TMAO precursors or TMAO predicting species. Supplementary Data 2b. Associations of intakes of choline and red meat, 34 or other TMAO precursors with TMAO levels, stratified by TMAO- producer status, additionally adjusted for other TMAO precursors. Supplementary Data 2c. Associations of intakes of choline and red meat, 37 or other TMAO precursors with TMAO levels, stratified by TMAO- producer status, in 6 different sensitivity analysis models. Supplementary Data 2d. Associations of intakes of red meat with HDLC 39 and HBA1c levels, stratified by TMAO-producer status or TMAO- associated abundant species. Supplementary Data 3a. Association of DNA gene clusters within 40 TMAO-associated species with plasma TMAO levels, and intakes of choline and red meat. Supplementary Data 3b. Association of transcriptions of gene clusters 46 (RNA/DNA ratio) within TMAO-associated species with plasma TMAO levels, and intakes of choline and red meat. Supplementary Data 4a. Association of DNA enzyme within TMAO- 62 associated species with plasma TMAO levels, and intakes of choline and red meat. -

Ordered Association of Helicase Loader Proteins with the Bacillus Subtilis Origin of Replication in Vivo
Ordered association of helicase loader proteins with the Bacillus subtilis origin of replication in vivo The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation Smits, Wiep Klaas, Alexi I. Goranov, and Alan D. Grossman. “Ordered association of helicase loader proteins with the Bacillus subtilis origin of replication in vivo.” Molecular Microbiology 75.2 (2010): 452–461. As Published http://dx.doi.org/10.1111/j.1365-2958.2009.06999.x Publisher Wiley Blackwell (Blackwell Publishing) Version Author's final manuscript Citable link http://hdl.handle.net/1721.1/73616 Terms of Use Creative Commons Attribution-Noncommercial-Share Alike 3.0 Detailed Terms http://creativecommons.org/licenses/by-nc-sa/3.0/ NIH Public Access Author Manuscript Mol Microbiol. Author manuscript; available in PMC 2011 January 1. NIH-PA Author ManuscriptPublished NIH-PA Author Manuscript in final edited NIH-PA Author Manuscript form as: Mol Microbiol. 2010 January ; 75(2): 452±461. doi:10.1111/j.1365-2958.2009.06999.x. Ordered association of helicase loader proteins with the Bacillus subtilis origin of replication in vivo Wiep Klaas Smits, Alexi I. Goranov, and Alan D. Grossman* Department of Biology, Massachusetts Institute of Technology, Cambridge, MA 02139 Summary The essential proteins DnaB, DnaD, and DnaI of Bacillus subtilis are required for initiation, but not elongation, of DNA replication, and for replication restart at stalled forks. The interactions and functions of these proteins have largely been determined in vitro based on their roles in replication restart. During replication initiation in vivo, it is not known if these proteins, and the replication initiator DnaA, associate with oriC independently of each other by virtue of their DNA binding activities, as a (sub)complex like other loader proteins, or in a particular dependent order. -

A Free-Living Protist That Lacks Canonical Eukaryotic DNA Replication and Segregation Systems
bioRxiv preprint doi: https://doi.org/10.1101/2021.03.14.435266; this version posted March 15, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 A free-living protist that lacks canonical eukaryotic DNA replication and segregation systems 2 Dayana E. Salas-Leiva1, Eelco C. Tromer2,3, Bruce A. Curtis1, Jon Jerlström-Hultqvist1, Martin 3 Kolisko4, Zhenzhen Yi5, Joan S. Salas-Leiva6, Lucie Gallot-Lavallée1, Geert J. P. L. Kops3, John M. 4 Archibald1, Alastair G. B. Simpson7 and Andrew J. Roger1* 5 1Centre for Comparative Genomics and Evolutionary Bioinformatics (CGEB), Department of 6 Biochemistry and Molecular Biology, Dalhousie University, Halifax, NS, Canada, B3H 4R2 2 7 Department of Biochemistry, University of Cambridge, Cambridge, United Kingdom 8 3Oncode Institute, Hubrecht Institute – KNAW (Royal Netherlands Academy of Arts and Sciences) 9 and University Medical Centre Utrecht, Utrecht, The Netherlands 10 4Institute of Parasitology Biology Centre, Czech Acad. Sci, České Budějovice, Czech Republic 11 5Guangzhou Key Laboratory of Subtropical Biodiversity and Biomonitoring, School of Life Science, 12 South China Normal University, Guangzhou 510631, China 13 6CONACyT-Centro de Investigación en Materiales Avanzados, Departamento de medio ambiente y 14 energía, Miguel de Cervantes 120, Complejo Industrial Chihuahua, 31136 Chihuahua, Chih., México 15 7Centre for Comparative Genomics and Evolutionary Bioinformatics (CGEB), Department of 16 Biology, Dalhousie University, Halifax, NS, Canada, B3H 4R2 17 *corresponding author: [email protected] 18 D.E.S-L ORCID iD: 0000-0003-2356-3351 19 E.C.T. -

Product Sheet Info
Master Clone List for NR-19278 Streptococcus pneumoniae, Strain TIGR4, Gateway® Clone Set, Recombinant in Escherichia coli, Plates 1-23 Catalog No. NR-19278 Table 1: Streptococcus pneumoniae, Strain TIGR4, Gateway® Clone, Plate 1 (YSPCA), NR-195681 Well ORF Accession Average Depth of Clone Locus ID Description Position Length Number Coverage 22040 A01 SP1028 hypothetical protein SP_1028 984 NP_345503.1 8.94410569 22062 A02 SP0684 hypothetical protein SP_0684 681 NP_345189.1 5.09985316 22066 A03 SP0747 hypothetical protein SP_0747 729 NP_345246.1 12.4828532 22067 A04 SP0934 hypothetical protein SP_0934 888 NP_345418.1 8.29617117 22068 A05 SP0612 hypothetical protein SP_0612 627 NP_345124.1 3.25039872 22074 A06 SP1678 hypothetical protein SP_1678 838 NP_346117.1 4.81622912 22075 A07 SP0699 hypothetical protein SP_0699 690 NP_345203.1 12.3768116 22076 A08 SP0195 hypothetical protein SP_0194 294 NP_344735.1 4.7755102 22080 A09 SP0821 hypothetical protein SP_0821 795 NP_345313.1 9.35345912 22081 A10 SP0407 hypothetical protein SP_0407 471 NP_344930.1 4.30997877 22083 A11 SP0070 hypothetical protein SP_0070 195 NP_344619.1 1.95384615 22086 A12 SP0654 hypothetical protein SP_0654 651 NP_345159.1 4.78341014 22089 B01 SP1120 hypothetical protein SP_1120 1104 NP_345591.1 10.7336957 22090 B02 SP0414 hypothetical protein SP_0414 474 NP_344937.1 4.80379747 22091 B03 SP1788 hypothetical protein SP_1788 169 NP_346221.1 1.6035503 22094 B04 SP0172 hypothetical protein SP_0172 270 NP_344713.1 14.9444444 22098 B05 SP0316 hypothetical protein SP_0316 381 -

Dnai DVD Transcripts
DNAi DVD 1 DNAi DVD Transcripts Selecting genes to patent Mark Adams a private company's approach to patenting genes It was never our intention to patent all the genes, all the genes aren't patentable, and the Patent Office has made that very clear, in fact, that they have stringent rules for what's patentable and what's not, and that neither the entire genome nor the entire set of genes would be patentable. So we've taken the same very selective approach to doing that, that is common in the biotechnology, pharmaceutical industries, as well as in academic universities and the NIH [National Institutes of Health]. After all, the NIH holds more gene patents than any other organization. It's sort of a bundle, the gene sequence and the protein and antibodies made from that protein are all typically part of a patent application, depending on what the commercial plan rationale is for it. Billions of bases Mark Adams there are 2.9 billion letters in the human genome In the human genome there are 2.9 billion As, Cs, Gs, and Ts. If you wanted to readout the genome sequence and could do that at ten bases a second, ACGT, ACGT, ACGT, it would take eleven years to read the genome sequence. It's a tremendous amount of information and if you print it up on a big wall poster, a tiny fraction of it, it's all As, Cs, Gs, and Ts. Mutations & cancer Bruce Ames cancer is caused by an accumulation of mutations So I think cancer is a disease of DNA. -

Helicase Loader Complex Reveals Insights Into the Mechanism of Bacterial Primosome Assembly
ARTICLE Received 20 Jun 2013 | Accepted 22 Aug 2013 | Published 19 Sep 2013 DOI: 10.1038/ncomms3495 OPEN Structure of a helicase–helicase loader complex reveals insights into the mechanism of bacterial primosome assembly Bin Liu1,2, William K. Eliason1,2 & Thomas A. Steitz1,2,3 During the assembly of the bacterial loader-dependent primosome, helicase loader proteins bind to the hexameric helicase ring, deliver it onto the oriC DNA and then dissociate from the complex. Here, to provide a better understanding of this key process, we report the crystal structure of the B570-kDa prepriming complex between the Bacillus subtilis loader protein and the Bacillus stearothermophilus helicase, as well as the helicase-binding domain of primase with a molar ratio of 6:6:3 at 7.5 Å resolution. The overall architecture of the complex exhibits a three-layered ring conformation. Moreover, the structure combined with the proposed model suggests that the shift from the ‘open-ring’ to the ‘open-spiral’ and then the ‘closed- spiral’ state of the helicase ring due to the binding of single-stranded DNA may be the cause of the loader release. 1 Department of Molecular Biophysics and Biochemistry, Yale University, New Haven, Connecticut 06520, USA. 2 Howard Hughes Medical Institute, New Haven, Connecticut 06510, USA. 3 Department of Chemistry, Yale University, New Haven, Connecticut 06520, USA. Correspondence and requests for materials should be addressed to T.A.S. (email: [email protected]). NATURE COMMUNICATIONS | 4:2495 | DOI: 10.1038/ncomms3495 | www.nature.com/naturecommunications 1 & 2013 Macmillan Publishers Limited. All rights reserved. -

Dcia Is an Ancestral Replicative Helicase Operator Essential for Bacterial Replication Initiation
ARTICLE Received 20 Apr 2016 | Accepted 15 Sep 2016 | Published 10 Nov 2016 DOI: 10.1038/ncomms13271 OPEN DciA is an ancestral replicative helicase operator essential for bacterial replication initiation Pierre Bre´zellec1,*, Isabelle Vallet-Gely2,*, Christophe Possoz2,*, Sophie Quevillon-Cheruel2 & Jean-Luc Ferat1,2 Delivery of the replicative helicase onto DNA is an essential step in the initiation of replication. In bacteria, DnaC (in Escherichia coli) and DnaI (in Bacillus subtilis) are repre- sentative of the two known mechanisms that assist the replicative helicase at this stage. Here, we establish that these two strategies cannot be regarded as prototypical of the bacterial domain since dnaC and dnaI (dna[CI]) are present in only a few bacterial phyla. We show that dna[CI] was domesticated at least seven times through evolution in bacteria and at the expense of one gene, which we rename dciA (dna[CI] antecedent), suggesting that DciA and Dna[CI] share a common function. We validate this hypothesis by establishing in Pseudomonas aeruginosa that DciA possesses the attributes of the replicative helicase-oper- ating proteins associated with replication initiation. 1 Universite de Versailles Saint-Quentin, 45 Avenue des Etats-Unis, Versailles 78000, France. 2 Institute for Integrative Biology of the Cell (I2BC), CEA, CNRS, Univ. Paris-Sud, Universite´ Paris-Saclay, Gif-sur-Yvette 91198, France. * These authors contributed equally to this work. Correspondence and requests for materials should be addressed to J.-L.F. (email: [email protected]). NATURE COMMUNICATIONS | 7:13271 | DOI: 10.1038/ncomms13271 | www.nature.com/naturecommunications 1 ARTICLE NATURE COMMUNICATIONS | DOI: 10.1038/ncomms13271 eplicative helicases are essential components of the Enterobacteriales because this order encompasses both the replication machinery. -

Mutational Analysis of Developmental Control in Caulobacter Crescentus
Proc. Nati. Acad. Sci. USA Vol. 74, No. 1, pp. 124-128, January 1977 Biochemistry Mutational analysis of developmental control in Caulobacter crescentus (cell cycle/flagellin synthesis/differentiation) MARY ANN OSLEY AND AUSTIN NEWTON Department of Biology, Princeton University, Princeton, New Jersey 08540 Communicated by J. T. Bonner, October 20,1976 ABSTRACT The relationship between the cell cycle and 19089), after irradiation with ultraviolet light to produce control of development has been studied by a genetic analysis 0.1-1.0% survival. Colonies from surviving cells were repli- of Caulobacter crescentus. The behavior of conditional cell division mutants showed that cell cycle events, such as DNA caprinted to Pye-agar and supplemented M3-agar plates and replication and cell division, are organized into a dependent incubated at 300 and 370 to identify temperature sensitive pathway(s), i.e., later.steps cannot proceed until earlier ones are mutants. completed. The ability of these strains to develop normally Screen of Mutant Phenotypes. Mutants were grown at 300 under nonpermissive conditions suggested that flagellin syn- to a density of 108 cells per ml in supplemented medium M3, thesis and stalk formation are dependent on the completion of and portions of the cultures were incubated at 300 and 370 for different cell-cycle steps: the periodicity of flagellin synthesis is achieved by coupling it to either DNA chain elongation or 6-9 hr. The cells were examined for stalk formation and flagella completion, and stalk formation is dependent on a later step in in the electron microscope after staining with 1% uranyl acetate the cell-division pathway. -

Loading Mechanisms of Ring Helicases at Replication Originsmmi 8012 6..16
Molecular Microbiology (2012) 84(1), 6–16 doi:10.1111/j.1365-2958.2012.08012.x First published online 15 March 2012 MicroReview Loading mechanisms of ring helicases at replication originsmmi_8012 6..16 Panos Soultanas* sequences. Separation of the two strands by ubiquitous School of Chemistry, Centre for Biomolecular Sciences, replicative DNA helicases reveals the parental nucleotide University of Nottingham, University Park, Nottingham sequences for DNA polymerases to copy into new NG7 2RD, UK. strands. Replicative helicases are closed ring structures with single DNA strands threaded through the ring during replication initiation via a process known as helicase Summary loading. Although the problem of threading a very long Threading of DNA through the central channel of a linear DNA molecule through a circular helicase ring is replicative ring helicase is known as helicase loading, fundamentally the same in all living organisms, a remark- and is a pivotal event during replication initiation at able variety of loading mechanisms have evolved utilizing replication origins. Once loaded, the helicase recruits the basic principles of ring opening or ring assembly. Here the primase through a direct protein–protein interac- we review recent advances in our understanding of tion to complete the initial ‘priming step’ of DNA helicase loading mechanisms in different organisms replication. Subsequent assembly of the polymerases (Table 1). We discuss and compare mechanisms with and processivity factors completes the structure of direct or indirect involvement of specialized helicase- the replisome. Two replisomes are assembled, one on loader proteins, as well as self-loading mechanisms in the each strand, and move in opposite directions to rep- absence of helicase loaders. -
Application of PCR to Detect Varieted Purity in Barley Malt by Debra Kay
Application of PCR to detect varieted purity in barley malt by Debra Kay Habernicht A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science in Agronomy Montana State University © Copyright by Debra Kay Habernicht (1997) Abstract: Genetic markers are used to detect inherited physical or molecular characteristics that differ among individuals. The field of forensic science has been revolutionized by the ability to extract and type DNA, from biological fluids left at a crime scene, using molecular techniques. These same techniques can be applied to the area of plant and seed identification. Currently, barley cultivar identification is based on morphological characteristics. The genetic diversity within the two-row and six-row germplasm is narrow and limits fhe usefulness of morphological characteristics. Also, once the barley is malted most of these morphological traits are undetectable. The North American Barley Genome Mapping Project (NABGMP) has generated large quantities of molecular markers for mapping. Most of the NABGMP clones have been sequenced and sequence-tagged-site (STS) specific primers developed for polymerase chain reaction (PCR)-based analysis. We used the STS-PCR approach to identify DNA fragments that differ in structure among genotypes. These primers with the use of endonucleases, provide a rapid analysis of DNA from young leaf tissue, harvested grain, or malted barley. Molecular markers were identified that differentiated cultivars within and between the two-row and six-row malting germplasm. We modified current DNA isolation protocols to have practical application in industry. These methods are described in detail. Using these techniques, cultivars were characterized for heterogeneity. -

A Xenopus Egg Factor with DNA-Binding Properties Characterisuc of Terminus- Specific Telomeric Proteins
Downloaded from genesdev.cshlp.org on October 9, 2021 - Published by Cold Spring Harbor Laboratory Press A Xenopus egg factor with DNA-binding properties characterisuc of terminus- specific telomeric proteins Maria E. Cardenas, 1 Alessandro Bianchi, and Titia de Lange 2 The Rockefeller University, New York, New York 10021-6399 USA We have identified a Xenopus laevis protein factor that specifically recognizes vertebrate telomeric repeats at DNA ends. This factor, called Xenopns telomere end factor (XTEF), is detected predominantly in extracts of Xenopus eggs and ovaries, which are estimated to contain sufficient XTEF to bind -3 x 107 DNA ends. In contrast, XTEF is much less abundant (-90 per cell} in extracts of somatic cell nuclei. Mobility retardation analysis of the XTEF activity in egg extracts indicates that this factor binds the vertebrate telomeric repeat sequence (TTAGGG)2 when present in a single-stranded 3' overhang. Single-stranded 3' extensions of (TTTGGG)2, (AAAGGG)2, (TTACCC)2, or a nonrepetitive sequence fail to bind XTEF efficiently, whereas changes in the double-stranded sequence 5' to the TTAGGG repeat tail are tolerated. TTAGGG repeats are not recognized at internal positions, at a 5' protruding end, or in double-stranded DNA. In addition, the factor does not bind RNA with single-stranded UUAGGG repeats at a 3' end. XTEF-DNA complexes form and are stable in high salt. The DNA-binding properties of XTEF resemble the characteristics of a class of terminus-specific telomere proteins identified previously in hypotrichous ciliates. [Key Words: Xenopus egg; single-stranded DNA-binding activity; telomeric DNA; vertebrate telomeres] Received February 2, 1993; revised version accepted March 15, 1993.