Loading Mechanisms of Ring Helicases at Replication Originsmmi 8012 6..16
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Curr.Opin.Chem. Biol., 15, 587-594
Available online at www.sciencedirect.com Bacterial replicases and related polymerases Charles S McHenry Bacterial replicases are complex, tripartite replicative replication that are conserved among all life forms are machines. They contain a polymerase, Pol III, a b2 processivity illustrated in Figure 1. factor and a DnaX complex ATPase that loads b2 onto DNA and chaperones Pol III onto the newly loaded b2. Many Structure and function of a, the catalytic bacteria encode both a full length t and a shorter g form of subunit DnaX by a variety of mechanisms. The polymerase catalytic Like all polymerases, Pol III a contains palm, thumb and subunit of Pol III, a, contains a PHP domain that not only binds fingers domains, in the shape of a cupped right hand 2+ to prototypical e Mg -dependent exonuclease, but also Figure 2. However, apo-enzyme structures of the full 2+ contains a second Zn -dependent proofreading length Thermus aquaticus (Taq) and a truncated version of exonuclease, at least in some bacteria. Replication of the E. coli (Eco) a subunit revealed a big surprise: the palm chromosomes of low GC Gram-positive bacteria require two domain has the basic fold of the X family of DNA Pol IIIs, one of which, DnaE, appears to extend RNA primers a polymerases, which includes the slow, non-processive only short distance before handing the product off to the major Pol bs [1 ,2 ]. replicase, PolC. Other bacteria encode a second Pol III (ImuC) that apparently replaces Pol V, required for induced A ternary complex of a dideoxy-terminated primer-tem- mutagenesis in E. -

A MUTYH Germline Mutation Is Associated with Small Intestinal
248 J P Dumanski et al. MUTYH substitution 24:8 427–443 Research Gly396Asp in SI-NETs Open Access A MUTYH germline mutation is associated with small intestinal neuroendocrine tumors Jan P Dumanski1,*, Chiara Rasi1,*, Peyman Björklund2, Hanna Davies1, Abir S Ali3, Malin Grönberg3, Staffan Welin3, Halfdan Sorbye4,5, Henning Grønbæk6, Janet L Cunningham7, Lars A Forsberg1, Lars Lind8, Erik Ingelsson9, Peter Stålberg10, Per Hellman10 and Eva Tiensuu Janson3 1Department of Immunology, Genetics and Pathology and SciLifeLab, Uppsala University, Uppsala, Sweden 2Department of Surgical Sciences, Experimental Surgery, Uppsala University, Uppsala, Sweden 3Department of Medical Sciences, Endocrine Oncology, Uppsala University, Uppsala, Sweden 4Department of Oncology, Haukeland University Hospital, Bergen, Norway 5Department of Clinical Science, University of Bergen, Bergen, Norway 6Department of Hepatology and Gastroenterology, Aarhus University Hospital, Aarhus, Denmark 7 Department of Neuroscience, Uppsala University, Uppsala, Sweden Correspondence 8 Department of Medical Sciences, Uppsala University, Uppsala, Sweden should be addressed 9 Division of Cardiovascular Medicine, Department of Medicine, Stanford University, San Francisco, California, USA to E Tiensuu Janson 10 Department of Surgical Sciences, Endocrine Surgery, Uppsala University, Uppsala, Sweden Email *(J P Dumanski and C Rasi shared first co-authorship) eva.tiensuu_janson@medsci. uu.se Abstract The genetics behind predisposition to small intestinal neuroendocrine tumors (SI-NETs) Key Words Endocrine-Related Cancer Endocrine-Related is largely unknown, but there is growing awareness of a familial form of the disease. f familial cancer We aimed to identify germline mutations involved in the carcinogenesis of SI-NETs. f cancer predisposition The strategy included next-generation sequencing of exome- and/or whole-genome of f small intestinal carcinoid blood DNA, and in selected cases, tumor DNA, from 24 patients from 15 families with f oxidative stress the history of SI-NETs. -

Conversion of OX174 and Fd Single-Stranded DNA to Replicative Forms in Extracts of Escherichia Coli (Dnac, Dnad, and Dnag Gene Products/DNA Polymerase III) REED B
Proc. Nat. Acad. Sci. USA Vol. 69, No. 11, pp. 3233-3237, November 1972 Conversion of OX174 and fd Single-Stranded DNA to Replicative Forms in Extracts of Escherichia coli (dnaC, dnaD, and dnaG gene products/DNA polymerase III) REED B. WICKNER, MICHEL WRIGHT, SUE WICKNER, AND JERARD HURWITZ Department of Developmental Biology and Cancer, Division of Biological Sciences, Albert Einstein College of Medicine, Bronx, New York 10461 Communicated by Harry Eagle, August 28, 1972 ABSTRACT 4X174 and M13 (fd) single-stranded cir- MATERIALS AND METHODS cular DNAs are converted to their replicative forms by ex- tracts of E. coli pol Al cells. We find that the qX174 DNA- [a-32P]dTTP was obtained from New England Nuclear Corp. dependent reaction requires Mg++, ATP, and all four de- OX174 DNA was prepared by the method of Sinsheimer (7) oxynucleoside triphosphates, but not CTP, UTP, or GTP. or Franke and Ray (8), while fd viral DNA was prepared as This reaction also involves the products of the dnaC, dnaD, dnaE (DNA polymerase III), and dnaG genes, described (9). Pancreatic RNase was the highest grade ob- but not that of dnaF (ribonucleotide reductase). The in tainable from Worthington Biochemical Corp. It was further vitro conversion of fd single-stranded DNA to the replica- freed of possible contaminating DNase by heating a solution tive form requires all four ribonucleoside triphosphates, (2 mg/ml in 15 mM sodium citrate, pH 5) at 800 for 10 min. Mg++, and all four deoxynucleoside triphosphates. The E. protein was purified from E. coli strain reaction involves the product of gene dnaE but not those coli unwinding of genes dnaC, dnaD, dnaF, or dnaG. -
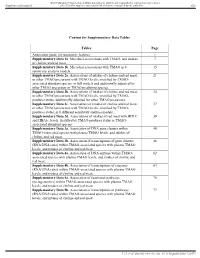
Supplementary Data TMAO Microbiome R1
BMJ Publishing Group Limited (BMJ) disclaims all liability and responsibility arising from any reliance Supplemental material placed on this supplemental material which has been supplied by the author(s) Gut Content for Supplementary Data Tables Tables Page Annotation guide for taxonomic features. 1 Supplementary Data 1a. Microbial associations with TMAO, and intakes 7 of choline and red meat. Supplementary Data 1b. Microbial associations with TMAO in 8 15 sensitivity analysis models. Supplementary Data 2a. Associations of intakes of choline and red meat, 21 or other TMAO precursors with TMAO levels, stratified by TMAO- associated abundant species, in full models and additionally adjusted for other TMAO precursors or TMAO predicting species. Supplementary Data 2b. Associations of intakes of choline and red meat, 34 or other TMAO precursors with TMAO levels, stratified by TMAO- producer status, additionally adjusted for other TMAO precursors. Supplementary Data 2c. Associations of intakes of choline and red meat, 37 or other TMAO precursors with TMAO levels, stratified by TMAO- producer status, in 6 different sensitivity analysis models. Supplementary Data 2d. Associations of intakes of red meat with HDLC 39 and HBA1c levels, stratified by TMAO-producer status or TMAO- associated abundant species. Supplementary Data 3a. Association of DNA gene clusters within 40 TMAO-associated species with plasma TMAO levels, and intakes of choline and red meat. Supplementary Data 3b. Association of transcriptions of gene clusters 46 (RNA/DNA ratio) within TMAO-associated species with plasma TMAO levels, and intakes of choline and red meat. Supplementary Data 4a. Association of DNA enzyme within TMAO- 62 associated species with plasma TMAO levels, and intakes of choline and red meat. -

Consequences and Resolution of Transcription–Replication Conflicts
life Review Consequences and Resolution of Transcription–Replication Conflicts Maxime Lalonde †, Manuel Trauner †, Marcel Werner † and Stephan Hamperl * Institute of Epigenetics and Stem Cells (IES), Helmholtz Zentrum München, 81377 Munich, Germany; [email protected] (M.L.); [email protected] (M.T.); [email protected] (M.W.) * Correspondence: [email protected] † These authors contributed equally. Abstract: Transcription–replication conflicts occur when the two critical cellular machineries respon- sible for gene expression and genome duplication collide with each other on the same genomic location. Although both prokaryotic and eukaryotic cells have evolved multiple mechanisms to coordinate these processes on individual chromosomes, it is now clear that conflicts can arise due to aberrant transcription regulation and premature proliferation, leading to DNA replication stress and genomic instability. As both are considered hallmarks of aging and human diseases such as cancer, understanding the cellular consequences of conflicts is of paramount importance. In this article, we summarize our current knowledge on where and when collisions occur and how these en- counters affect the genome and chromatin landscape of cells. Finally, we conclude with the different cellular pathways and multiple mechanisms that cells have put in place at conflict sites to ensure the resolution of conflicts and accurate genome duplication. Citation: Lalonde, M.; Trauner, M.; Keywords: transcription–replication conflicts; genomic instability; R-loops; torsional stress; common Werner, M.; Hamperl, S. fragile sites; early replicating fragile sites; replication stress; chromatin; fork reversal; MIDAS; G-MiDS Consequences and Resolution of Transcription–Replication Conflicts. Life 2021, 11, 637. https://doi.org/ 10.3390/life11070637 1. -

The Functional Analysis of Yaba, Which Interacts with Dnaa and Regulates Initiation of Chromosome Replication in Bacillus Subtils
Genes Genet. Syst. (2008) 83, p. 111–125 The functional analysis of YabA, which interacts with DnaA and regulates initiation of chromosome replication in Bacillus subtils Eunha Cho, Naotake Ogasawara and Shu Ishikawa* Graduate School of Information Science, Nara Institute of Science and Technology, 8916-5 Takayama, Ikoma, Nara 630-0101, Japan (Received 15 January 2008, accepted 1 February 2008) The initiation of bacterial chromosome DNA replication and its regulation are critical events. DnaA is essential for initiation of DNA replication and is con- served throughout bacteria. In Escherichia coli, hydrolysis of ATP-DnaA is pro- moted by Hda through formation of a ternary complex with DnaA and DnaN, ensuring the timely inactivation of DnaA during the replication cycle. In Bacillus subtilis, YabA also forms a ternary complex with DnaA and DnaN, and negatively regulates the initiation step of DNA replication. However, YabA shares no struc- tural homology with Hda and the regulatory mechanism itself has not been clarified. Here, in contrast to Hda, we observed that dnaA transcription was stable during under- and overexpression of YabA. ChAP-chip assays showed that the depletion of YabA did not affect DNA binding by DnaA. On the other hand, yeast two-hybrid analysis indicated that the DnaA ATP-binding domain interacts with YabA. Moreover, mutations in YabA interaction-deficient mutants, isolated by yeast two-hybrid analysis, are located at the back of the ATP-binding domain, whereas Hda is thought to interact with the ATP-binding pocket itself. The intro- duction into B. subtilis of a dnaAY144C mutation, which disabled the interaction with YabA but did not affect interactions either with DnaA itself or with DnaD, resulted in over-initiation and asynchronous initiation of replication and disabled the formation of YabA foci, further demonstrating that the amino acid on the oppo- site side to the ATP-binding pocket is important for YabA binding. -

Ordered Association of Helicase Loader Proteins with the Bacillus Subtilis Origin of Replication in Vivo
Ordered association of helicase loader proteins with the Bacillus subtilis origin of replication in vivo The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation Smits, Wiep Klaas, Alexi I. Goranov, and Alan D. Grossman. “Ordered association of helicase loader proteins with the Bacillus subtilis origin of replication in vivo.” Molecular Microbiology 75.2 (2010): 452–461. As Published http://dx.doi.org/10.1111/j.1365-2958.2009.06999.x Publisher Wiley Blackwell (Blackwell Publishing) Version Author's final manuscript Citable link http://hdl.handle.net/1721.1/73616 Terms of Use Creative Commons Attribution-Noncommercial-Share Alike 3.0 Detailed Terms http://creativecommons.org/licenses/by-nc-sa/3.0/ NIH Public Access Author Manuscript Mol Microbiol. Author manuscript; available in PMC 2011 January 1. NIH-PA Author ManuscriptPublished NIH-PA Author Manuscript in final edited NIH-PA Author Manuscript form as: Mol Microbiol. 2010 January ; 75(2): 452±461. doi:10.1111/j.1365-2958.2009.06999.x. Ordered association of helicase loader proteins with the Bacillus subtilis origin of replication in vivo Wiep Klaas Smits, Alexi I. Goranov, and Alan D. Grossman* Department of Biology, Massachusetts Institute of Technology, Cambridge, MA 02139 Summary The essential proteins DnaB, DnaD, and DnaI of Bacillus subtilis are required for initiation, but not elongation, of DNA replication, and for replication restart at stalled forks. The interactions and functions of these proteins have largely been determined in vitro based on their roles in replication restart. During replication initiation in vivo, it is not known if these proteins, and the replication initiator DnaA, associate with oriC independently of each other by virtue of their DNA binding activities, as a (sub)complex like other loader proteins, or in a particular dependent order. -

A Free-Living Protist That Lacks Canonical Eukaryotic DNA Replication and Segregation Systems
bioRxiv preprint doi: https://doi.org/10.1101/2021.03.14.435266; this version posted March 15, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 A free-living protist that lacks canonical eukaryotic DNA replication and segregation systems 2 Dayana E. Salas-Leiva1, Eelco C. Tromer2,3, Bruce A. Curtis1, Jon Jerlström-Hultqvist1, Martin 3 Kolisko4, Zhenzhen Yi5, Joan S. Salas-Leiva6, Lucie Gallot-Lavallée1, Geert J. P. L. Kops3, John M. 4 Archibald1, Alastair G. B. Simpson7 and Andrew J. Roger1* 5 1Centre for Comparative Genomics and Evolutionary Bioinformatics (CGEB), Department of 6 Biochemistry and Molecular Biology, Dalhousie University, Halifax, NS, Canada, B3H 4R2 2 7 Department of Biochemistry, University of Cambridge, Cambridge, United Kingdom 8 3Oncode Institute, Hubrecht Institute – KNAW (Royal Netherlands Academy of Arts and Sciences) 9 and University Medical Centre Utrecht, Utrecht, The Netherlands 10 4Institute of Parasitology Biology Centre, Czech Acad. Sci, České Budějovice, Czech Republic 11 5Guangzhou Key Laboratory of Subtropical Biodiversity and Biomonitoring, School of Life Science, 12 South China Normal University, Guangzhou 510631, China 13 6CONACyT-Centro de Investigación en Materiales Avanzados, Departamento de medio ambiente y 14 energía, Miguel de Cervantes 120, Complejo Industrial Chihuahua, 31136 Chihuahua, Chih., México 15 7Centre for Comparative Genomics and Evolutionary Bioinformatics (CGEB), Department of 16 Biology, Dalhousie University, Halifax, NS, Canada, B3H 4R2 17 *corresponding author: [email protected] 18 D.E.S-L ORCID iD: 0000-0003-2356-3351 19 E.C.T. -

Product Sheet Info
Master Clone List for NR-19278 Streptococcus pneumoniae, Strain TIGR4, Gateway® Clone Set, Recombinant in Escherichia coli, Plates 1-23 Catalog No. NR-19278 Table 1: Streptococcus pneumoniae, Strain TIGR4, Gateway® Clone, Plate 1 (YSPCA), NR-195681 Well ORF Accession Average Depth of Clone Locus ID Description Position Length Number Coverage 22040 A01 SP1028 hypothetical protein SP_1028 984 NP_345503.1 8.94410569 22062 A02 SP0684 hypothetical protein SP_0684 681 NP_345189.1 5.09985316 22066 A03 SP0747 hypothetical protein SP_0747 729 NP_345246.1 12.4828532 22067 A04 SP0934 hypothetical protein SP_0934 888 NP_345418.1 8.29617117 22068 A05 SP0612 hypothetical protein SP_0612 627 NP_345124.1 3.25039872 22074 A06 SP1678 hypothetical protein SP_1678 838 NP_346117.1 4.81622912 22075 A07 SP0699 hypothetical protein SP_0699 690 NP_345203.1 12.3768116 22076 A08 SP0195 hypothetical protein SP_0194 294 NP_344735.1 4.7755102 22080 A09 SP0821 hypothetical protein SP_0821 795 NP_345313.1 9.35345912 22081 A10 SP0407 hypothetical protein SP_0407 471 NP_344930.1 4.30997877 22083 A11 SP0070 hypothetical protein SP_0070 195 NP_344619.1 1.95384615 22086 A12 SP0654 hypothetical protein SP_0654 651 NP_345159.1 4.78341014 22089 B01 SP1120 hypothetical protein SP_1120 1104 NP_345591.1 10.7336957 22090 B02 SP0414 hypothetical protein SP_0414 474 NP_344937.1 4.80379747 22091 B03 SP1788 hypothetical protein SP_1788 169 NP_346221.1 1.6035503 22094 B04 SP0172 hypothetical protein SP_0172 270 NP_344713.1 14.9444444 22098 B05 SP0316 hypothetical protein SP_0316 381 -

Mechanism of Staphylococcal Multiresistance Plasmid Replication Origin Assembly by the Repa Protein
Mechanism of staphylococcal multiresistance plasmid replication origin assembly by the RepA protein Maria A. Schumachera,1, Nam K. Tonthata, Stephen M. Kwongb, Naga babu Chinnama, Michael A. Liub, Ronald A. Skurrayb, and Neville Firthb aDepartment of Biochemistry, Duke University Medical Center, Durham, NC 27710; and bSchool of Biological Sciences, University of Sydney, Sydney, NSW 2006, Australia Edited by James M. Berger, The Johns Hopkins University School of Medicine, Baltimore, MD, and approved May 15, 2014 (received for review April 1, 2014) The staphylococcal multiresistance plasmids are key contributors (16). In Gram-negative bacteria the primosome includes DnaG to the alarming rise in bacterial multidrug resistance. A conserved primase, the replicative helicase (DnaB), and DnaC (17). Rep- replication initiator, RepA, encoded on these plasmids is essential lication initiation in Gram-positive bacteria involves DnaG pri- for their propagation. RepA proteins consist of flexibly linked mase and helicase (DnaC) and the proteins DnaD, DnaI, and N-terminal (NTD) and C-terminal (CTD) domains. Despite their essen- DnaB (18–22). DnaD binds first to DnaA at the origin. This is tial role in replication, the molecular basis for RepA function is followed by binding of DnaI/DnaB and DnaG, which together unknown. Here we describe a complete structural and functional recruit the replicative helicase (23, 24). Instead of DnaA, plas- dissection of RepA proteins. Unexpectedly, both the RepA NTD and mids encode and use their own specific replication initiator CTD show similarity to the corresponding domains of the bacterial binding protein. Structures are only available for RepA proteins primosome protein, DnaD. Although the RepA and DnaD NTD both (F, R6K, and pPS10 Rep) harbored in Gram-negative bacteria. -

Dnai DVD Transcripts
DNAi DVD 1 DNAi DVD Transcripts Selecting genes to patent Mark Adams a private company's approach to patenting genes It was never our intention to patent all the genes, all the genes aren't patentable, and the Patent Office has made that very clear, in fact, that they have stringent rules for what's patentable and what's not, and that neither the entire genome nor the entire set of genes would be patentable. So we've taken the same very selective approach to doing that, that is common in the biotechnology, pharmaceutical industries, as well as in academic universities and the NIH [National Institutes of Health]. After all, the NIH holds more gene patents than any other organization. It's sort of a bundle, the gene sequence and the protein and antibodies made from that protein are all typically part of a patent application, depending on what the commercial plan rationale is for it. Billions of bases Mark Adams there are 2.9 billion letters in the human genome In the human genome there are 2.9 billion As, Cs, Gs, and Ts. If you wanted to readout the genome sequence and could do that at ten bases a second, ACGT, ACGT, ACGT, it would take eleven years to read the genome sequence. It's a tremendous amount of information and if you print it up on a big wall poster, a tiny fraction of it, it's all As, Cs, Gs, and Ts. Mutations & cancer Bruce Ames cancer is caused by an accumulation of mutations So I think cancer is a disease of DNA. -

The Primosomal Protein Dnad Inhibits Cooperative DNA Binding by the Replication Initiator Dnaa in Bacillus Subtilis
The primosomal protein DnaD inhibits cooperative DNA binding by the replication initiator DnaA in Bacillus subtilis The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation Bonilla, C. Y., and A. D. Grossman. “The Primosomal Protein DnaD Inhibits Cooperative DNA Binding by the Replication Initiator DnaA in Bacillus Subtilis.” Journal of Bacteriology 194.18 (2012): 5110–5117. As Published http://dx.doi.org/10.1128/jb.00958-12 Publisher American Society for Microbiology Version Author's final manuscript Citable link http://hdl.handle.net/1721.1/74629 Terms of Use Creative Commons Attribution-Noncommercial-Share Alike 3.0 Detailed Terms http://creativecommons.org/licenses/by-nc-sa/3.0/ Bonilla and Grossman 1 1 2 The primosomal protein DnaD inhibits cooperative DNA binding by the 3 replication initiator DnaA in Bacillus subtilis 4 5 6 Carla Y. Bonilla and Alan D. Grossman* 7 Department of Biology 8 Massachusetts Institute of Technology 9 Cambridge, MA 02139 10 11 Running title: Primosomal protein DnaD affects DNA binding by DnaA 12 13 14 15 *Corresponding author: 16 Department of Biology 17 Building 68-530 18 Massachusetts Institute of Technology 19 Cambridge, MA 02139 20 phone: 617-253-1515 21 fax: 617-253-2643 22 E-mail: [email protected] 23 24 25 26 Bonilla and Grossman 2 27 Abstract 28 DnaA is a AAA+ ATPase and the conserved replication initiator in bacteria. Bacteria control 29 the timing of replication initiation by regulating the activity of DnaA. DnaA binds to multiple 30 sites in the origin of replication (oriC) and is required for recruitment of proteins needed to load 31 the replicative helicase.