Control of Initiation of DNA Replication in Bacillus Subtilis and Escherichia Coli
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Replisome Assembly at Oric, the Replication Origin of E. Coli, Reveals an Explanation for Initiation Sites Outside an Origin
Molecular Cell, Vol. 4, 541±553, October, 1999, Copyright 1999 by Cell Press Replisome Assembly at oriC, the Replication Origin of E. coli, Reveals an Explanation for Initiation Sites outside an Origin Linhua Fang,*§ Megan J. Davey,² and Mike O'Donnell²³ have not been addressed. For example, is the local un- *Microbiology Department winding sufficiently large for two helicases to assemble Joan and Sanford I. Weill Graduate School of Medical for bidirectional replication, or does one helicase need Sciences of Cornell University to enter first and expand the bubble via helicase action New York, New York 10021 to make room for the second helicase? The known rep- ² The Rockefeller University and licative helicases are hexameric and encircle ssDNA. Howard Hughes Medical Institute Which strand does the initial helicase(s) at the origin New York, New York 10021 encircle, and if there are two, how are they positioned relative to one another? Primases generally require at least transient interaction with helicase to function. Can Summary primase function with the helicase(s) directly after heli- case assembly at the origin, or must helicase-catalyzed This study outlines the events downstream of origin DNA unwinding occur prior to RNA primer synthesis? unwinding by DnaA, leading to assembly of two repli- Chromosomal replicases are comprised of a ring-shaped cation forks at the E. coli origin, oriC. We show that protein clamp that encircles DNA, a clamp-loading com- two hexamers of DnaB assemble onto the opposing plex that uses ATP to assemble the clamp around DNA, strands of the resulting bubble, expanding it further, and a DNA polymerase that binds the circular clamp, yet helicase action is not required. -

Curr.Opin.Chem. Biol., 15, 587-594
Available online at www.sciencedirect.com Bacterial replicases and related polymerases Charles S McHenry Bacterial replicases are complex, tripartite replicative replication that are conserved among all life forms are machines. They contain a polymerase, Pol III, a b2 processivity illustrated in Figure 1. factor and a DnaX complex ATPase that loads b2 onto DNA and chaperones Pol III onto the newly loaded b2. Many Structure and function of a, the catalytic bacteria encode both a full length t and a shorter g form of subunit DnaX by a variety of mechanisms. The polymerase catalytic Like all polymerases, Pol III a contains palm, thumb and subunit of Pol III, a, contains a PHP domain that not only binds fingers domains, in the shape of a cupped right hand 2+ to prototypical e Mg -dependent exonuclease, but also Figure 2. However, apo-enzyme structures of the full 2+ contains a second Zn -dependent proofreading length Thermus aquaticus (Taq) and a truncated version of exonuclease, at least in some bacteria. Replication of the E. coli (Eco) a subunit revealed a big surprise: the palm chromosomes of low GC Gram-positive bacteria require two domain has the basic fold of the X family of DNA Pol IIIs, one of which, DnaE, appears to extend RNA primers a polymerases, which includes the slow, non-processive only short distance before handing the product off to the major Pol bs [1 ,2 ]. replicase, PolC. Other bacteria encode a second Pol III (ImuC) that apparently replaces Pol V, required for induced A ternary complex of a dideoxy-terminated primer-tem- mutagenesis in E. -

USP7 Couples DNA Replication Termination to Mitotic Entry
bioRxiv preprint doi: https://doi.org/10.1101/305318; this version posted April 20, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. USP7 couples DNA replication termination to mitotic entry Antonio Galarreta1*, Emilio Lecona1*, Pablo Valledor1, Patricia Ubieto1,2, Vanesa Lafarga1, Julia Specks1 & Oscar Fernandez-Capetillo1,3 1Genomic Instability Group, Spanish National Cancer Research Centre (CNIO), Madrid 28029, Spain 2Current Address: DNA Replication Group, Spanish National Cancer Research Centre (CNIO), Madrid 28029, Spain 3Science for Life Laboratory, Division of Genome Biology, Department of Medical Biochemistry and Biophysics, Karolinska Institute, S-171 21 Stockholm, Sweden *Co-first authors Correspondence: E.L. ([email protected]) or O.F. ([email protected]) Lead Contact: Oscar Fernandez-Capetillo Spanish National Cancer Research Centre (CNIO) Melchor Fernandez Almagro, 3 Madrid 28029, Spain Tel.: +34.91.732.8000 Ext: 3480 Fax: +34.91.732.8028 Email: [email protected] KEYWORDS: USP7; CDK1; DNA REPLICATION; MITOSIS; S/M TRANSITION. bioRxiv preprint doi: https://doi.org/10.1101/305318; this version posted April 20, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. USP7 coordinates the S/M transition 2 SUMMARY To ensure a faithful segregation of chromosomes, DNA must be fully replicated before mitotic entry. However, how cells sense the completion of DNA replication and to what extent this is linked to the activation of the mitotic machinery remains poorly understood. We previously showed that USP7 is a replisome-associated deubiquitinase with an essential role in DNA replication. -

A MUTYH Germline Mutation Is Associated with Small Intestinal
248 J P Dumanski et al. MUTYH substitution 24:8 427–443 Research Gly396Asp in SI-NETs Open Access A MUTYH germline mutation is associated with small intestinal neuroendocrine tumors Jan P Dumanski1,*, Chiara Rasi1,*, Peyman Björklund2, Hanna Davies1, Abir S Ali3, Malin Grönberg3, Staffan Welin3, Halfdan Sorbye4,5, Henning Grønbæk6, Janet L Cunningham7, Lars A Forsberg1, Lars Lind8, Erik Ingelsson9, Peter Stålberg10, Per Hellman10 and Eva Tiensuu Janson3 1Department of Immunology, Genetics and Pathology and SciLifeLab, Uppsala University, Uppsala, Sweden 2Department of Surgical Sciences, Experimental Surgery, Uppsala University, Uppsala, Sweden 3Department of Medical Sciences, Endocrine Oncology, Uppsala University, Uppsala, Sweden 4Department of Oncology, Haukeland University Hospital, Bergen, Norway 5Department of Clinical Science, University of Bergen, Bergen, Norway 6Department of Hepatology and Gastroenterology, Aarhus University Hospital, Aarhus, Denmark 7 Department of Neuroscience, Uppsala University, Uppsala, Sweden Correspondence 8 Department of Medical Sciences, Uppsala University, Uppsala, Sweden should be addressed 9 Division of Cardiovascular Medicine, Department of Medicine, Stanford University, San Francisco, California, USA to E Tiensuu Janson 10 Department of Surgical Sciences, Endocrine Surgery, Uppsala University, Uppsala, Sweden Email *(J P Dumanski and C Rasi shared first co-authorship) eva.tiensuu_janson@medsci. uu.se Abstract The genetics behind predisposition to small intestinal neuroendocrine tumors (SI-NETs) Key Words Endocrine-Related Cancer Endocrine-Related is largely unknown, but there is growing awareness of a familial form of the disease. f familial cancer We aimed to identify germline mutations involved in the carcinogenesis of SI-NETs. f cancer predisposition The strategy included next-generation sequencing of exome- and/or whole-genome of f small intestinal carcinoid blood DNA, and in selected cases, tumor DNA, from 24 patients from 15 families with f oxidative stress the history of SI-NETs. -

Roles of Methylation and Sequestration in the Mechanisms of DNA Replication in Some Members of the Enterobacteriaceae Family
Chapter 12 Roles of Methylation and Sequestration in the Mechanisms of DNA Replication in some Members of the Enterobacteriaceae Family Amine Aloui, Alya El May, Saloua Kouass Sahbani and Ahmed Landoulsi Additional information is available at the end of the chapter http://dx.doi.org/10.5772/51724 1. Introduction When growing cells divide, they need to copy their genetic material and distribute it to en‐ sure that each daughter cell receives one copy. This is a challenging task especially when the enormous length of the DNA compared to the cell size is considered. During DNA replica‐ tion, organization of the chromosomes is even more demanding, since replication forks con‐ tinuously produce new DNA. This DNA contains all the information required to build the cells and tissues of a prokaryotic or an eukaryotic organism. The exact replication of this in‐ formation in any species assures its genetic continuity from generation to generation and is critical to the normal development of an individual. The information stored in DNA is ar‐ ranged in hereditary units known as genes that control the identifiable traits of an organism. Discovery of the structure of DNA and subsequent elucidation of how DNA directs synthe‐ sis of RNA, which then directs assembly of proteins -the so-called central dogma - were monumental achievements that marked the early days of molecular biology. However, the simplified representation of the central dogma as DNA → RNA → protein does not reflect the role of proteins in the synthesis of nucleic acids. Moreover, proteins are largely responsi‐ ble for regulating DNA replication and gene expression, the entire process whereby the in‐ formation encoded in DNA is decoded into the proteins that characterize various cell types. -

Polymerase Δ Deficiency Causes Syndromic Immunodeficiency with Replicative Stress
Polymerase δ deficiency causes syndromic immunodeficiency with replicative stress Cecilia Domínguez Conde, … , Mirjam van der Burg, Kaan Boztug J Clin Invest. 2019. https://doi.org/10.1172/JCI128903. Research Article Genetics Immunology Graphical abstract Find the latest version: https://jci.me/128903/pdf The Journal of Clinical Investigation RESEARCH ARTICLE Polymerase δ deficiency causes syndromic immunodeficiency with replicative stress Cecilia Domínguez Conde,1,2 Özlem Yüce Petronczki,1,2,3 Safa Baris,4,5 Katharina L. Willmann,1,2 Enrico Girardi,2 Elisabeth Salzer,1,2,3,6 Stefan Weitzer,7 Rico Chandra Ardy,1,2,3 Ana Krolo,1,2,3 Hanna Ijspeert,8 Ayca Kiykim,4,5 Elif Karakoc-Aydiner,4,5 Elisabeth Förster-Waldl,9 Leo Kager,6 Winfried F. Pickl,10 Giulio Superti-Furga,2,11 Javier Martínez,7 Joanna I. Loizou,2 Ahmet Ozen,4,5 Mirjam van der Burg,8 and Kaan Boztug1,2,3,6 1Ludwig Boltzmann Institute for Rare and Undiagnosed Diseases, 2CeMM Research Center for Molecular Medicine of the Austrian Academy of Sciences, and 3St. Anna Children’s Cancer Research Institute (CCRI), Vienna, Austria. 4Pediatric Allergy and Immunology, Marmara University, Faculty of Medicine, Istanbul, Turkey. 5Jeffrey Modell Diagnostic Center for Primary Immunodeficiency Diseases, Marmara University, Istanbul, Turkey. 6St. Anna Children’s Hospital, Department of Pediatrics and Adolescent Medicine, Vienna, Austria. 7Center for Medical Biochemistry, Medical University of Vienna, Vienna, Austria. 8Department of Pediatrics, Laboratory for Immunology, Leiden University Medical Centre, Leiden, Netherlands. 9Department of Neonatology, Pediatric Intensive Care and Neuropediatrics, Department of Pediatrics and Adolescent Medicine, 10Institute of Immunology, Center for Pathophysiology, Infectiology and Immunology, and 11Center for Physiology and Pharmacology, Medical University of Vienna, Vienna, Austria. -

The Obscure World of Integrative and Mobilizable Elements Gérard Guédon, Virginie Libante, Charles Coluzzi, Sophie Payot-Lacroix, Nathalie Leblond-Bourget
The obscure world of integrative and mobilizable elements Gérard Guédon, Virginie Libante, Charles Coluzzi, Sophie Payot-Lacroix, Nathalie Leblond-Bourget To cite this version: Gérard Guédon, Virginie Libante, Charles Coluzzi, Sophie Payot-Lacroix, Nathalie Leblond-Bourget. The obscure world of integrative and mobilizable elements: Highly widespread elements that pirate bacterial conjugative systems. Genes, MDPI, 2017, 8 (11), pp.337. 10.3390/genes8110337. hal- 01686871 HAL Id: hal-01686871 https://hal.archives-ouvertes.fr/hal-01686871 Submitted on 26 May 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Distributed under a Creative Commons Attribution| 4.0 International License G C A T T A C G G C A T genes Review The Obscure World of Integrative and Mobilizable Elements, Highly Widespread Elements that Pirate Bacterial Conjugative Systems Gérard Guédon *, Virginie Libante, Charles Coluzzi, Sophie Payot and Nathalie Leblond-Bourget * ID DynAMic, Université de Lorraine, INRA, 54506 Vandœuvre-lès-Nancy, France; [email protected] (V.L.); [email protected] (C.C.); [email protected] (S.P.) * Correspondence: [email protected] (G.G.); [email protected] (N.L.-B.); Tel.: +33-037-274-5142 (G.G.); +33-037-274-5146 (N.L.-B.) Received: 12 October 2017; Accepted: 15 November 2017; Published: 22 November 2017 Abstract: Conjugation is a key mechanism of bacterial evolution that involves mobile genetic elements. -

Conversion of OX174 and Fd Single-Stranded DNA to Replicative Forms in Extracts of Escherichia Coli (Dnac, Dnad, and Dnag Gene Products/DNA Polymerase III) REED B
Proc. Nat. Acad. Sci. USA Vol. 69, No. 11, pp. 3233-3237, November 1972 Conversion of OX174 and fd Single-Stranded DNA to Replicative Forms in Extracts of Escherichia coli (dnaC, dnaD, and dnaG gene products/DNA polymerase III) REED B. WICKNER, MICHEL WRIGHT, SUE WICKNER, AND JERARD HURWITZ Department of Developmental Biology and Cancer, Division of Biological Sciences, Albert Einstein College of Medicine, Bronx, New York 10461 Communicated by Harry Eagle, August 28, 1972 ABSTRACT 4X174 and M13 (fd) single-stranded cir- MATERIALS AND METHODS cular DNAs are converted to their replicative forms by ex- tracts of E. coli pol Al cells. We find that the qX174 DNA- [a-32P]dTTP was obtained from New England Nuclear Corp. dependent reaction requires Mg++, ATP, and all four de- OX174 DNA was prepared by the method of Sinsheimer (7) oxynucleoside triphosphates, but not CTP, UTP, or GTP. or Franke and Ray (8), while fd viral DNA was prepared as This reaction also involves the products of the dnaC, dnaD, dnaE (DNA polymerase III), and dnaG genes, described (9). Pancreatic RNase was the highest grade ob- but not that of dnaF (ribonucleotide reductase). The in tainable from Worthington Biochemical Corp. It was further vitro conversion of fd single-stranded DNA to the replica- freed of possible contaminating DNase by heating a solution tive form requires all four ribonucleoside triphosphates, (2 mg/ml in 15 mM sodium citrate, pH 5) at 800 for 10 min. Mg++, and all four deoxynucleoside triphosphates. The E. protein was purified from E. coli strain reaction involves the product of gene dnaE but not those coli unwinding of genes dnaC, dnaD, dnaF, or dnaG. -
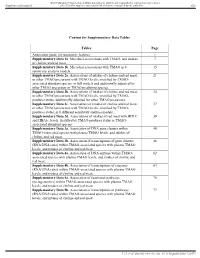
Supplementary Data TMAO Microbiome R1
BMJ Publishing Group Limited (BMJ) disclaims all liability and responsibility arising from any reliance Supplemental material placed on this supplemental material which has been supplied by the author(s) Gut Content for Supplementary Data Tables Tables Page Annotation guide for taxonomic features. 1 Supplementary Data 1a. Microbial associations with TMAO, and intakes 7 of choline and red meat. Supplementary Data 1b. Microbial associations with TMAO in 8 15 sensitivity analysis models. Supplementary Data 2a. Associations of intakes of choline and red meat, 21 or other TMAO precursors with TMAO levels, stratified by TMAO- associated abundant species, in full models and additionally adjusted for other TMAO precursors or TMAO predicting species. Supplementary Data 2b. Associations of intakes of choline and red meat, 34 or other TMAO precursors with TMAO levels, stratified by TMAO- producer status, additionally adjusted for other TMAO precursors. Supplementary Data 2c. Associations of intakes of choline and red meat, 37 or other TMAO precursors with TMAO levels, stratified by TMAO- producer status, in 6 different sensitivity analysis models. Supplementary Data 2d. Associations of intakes of red meat with HDLC 39 and HBA1c levels, stratified by TMAO-producer status or TMAO- associated abundant species. Supplementary Data 3a. Association of DNA gene clusters within 40 TMAO-associated species with plasma TMAO levels, and intakes of choline and red meat. Supplementary Data 3b. Association of transcriptions of gene clusters 46 (RNA/DNA ratio) within TMAO-associated species with plasma TMAO levels, and intakes of choline and red meat. Supplementary Data 4a. Association of DNA enzyme within TMAO- 62 associated species with plasma TMAO levels, and intakes of choline and red meat. -

Consequences and Resolution of Transcription–Replication Conflicts
life Review Consequences and Resolution of Transcription–Replication Conflicts Maxime Lalonde †, Manuel Trauner †, Marcel Werner † and Stephan Hamperl * Institute of Epigenetics and Stem Cells (IES), Helmholtz Zentrum München, 81377 Munich, Germany; [email protected] (M.L.); [email protected] (M.T.); [email protected] (M.W.) * Correspondence: [email protected] † These authors contributed equally. Abstract: Transcription–replication conflicts occur when the two critical cellular machineries respon- sible for gene expression and genome duplication collide with each other on the same genomic location. Although both prokaryotic and eukaryotic cells have evolved multiple mechanisms to coordinate these processes on individual chromosomes, it is now clear that conflicts can arise due to aberrant transcription regulation and premature proliferation, leading to DNA replication stress and genomic instability. As both are considered hallmarks of aging and human diseases such as cancer, understanding the cellular consequences of conflicts is of paramount importance. In this article, we summarize our current knowledge on where and when collisions occur and how these en- counters affect the genome and chromatin landscape of cells. Finally, we conclude with the different cellular pathways and multiple mechanisms that cells have put in place at conflict sites to ensure the resolution of conflicts and accurate genome duplication. Citation: Lalonde, M.; Trauner, M.; Keywords: transcription–replication conflicts; genomic instability; R-loops; torsional stress; common Werner, M.; Hamperl, S. fragile sites; early replicating fragile sites; replication stress; chromatin; fork reversal; MIDAS; G-MiDS Consequences and Resolution of Transcription–Replication Conflicts. Life 2021, 11, 637. https://doi.org/ 10.3390/life11070637 1. -

The Functional Analysis of Yaba, Which Interacts with Dnaa and Regulates Initiation of Chromosome Replication in Bacillus Subtils
Genes Genet. Syst. (2008) 83, p. 111–125 The functional analysis of YabA, which interacts with DnaA and regulates initiation of chromosome replication in Bacillus subtils Eunha Cho, Naotake Ogasawara and Shu Ishikawa* Graduate School of Information Science, Nara Institute of Science and Technology, 8916-5 Takayama, Ikoma, Nara 630-0101, Japan (Received 15 January 2008, accepted 1 February 2008) The initiation of bacterial chromosome DNA replication and its regulation are critical events. DnaA is essential for initiation of DNA replication and is con- served throughout bacteria. In Escherichia coli, hydrolysis of ATP-DnaA is pro- moted by Hda through formation of a ternary complex with DnaA and DnaN, ensuring the timely inactivation of DnaA during the replication cycle. In Bacillus subtilis, YabA also forms a ternary complex with DnaA and DnaN, and negatively regulates the initiation step of DNA replication. However, YabA shares no struc- tural homology with Hda and the regulatory mechanism itself has not been clarified. Here, in contrast to Hda, we observed that dnaA transcription was stable during under- and overexpression of YabA. ChAP-chip assays showed that the depletion of YabA did not affect DNA binding by DnaA. On the other hand, yeast two-hybrid analysis indicated that the DnaA ATP-binding domain interacts with YabA. Moreover, mutations in YabA interaction-deficient mutants, isolated by yeast two-hybrid analysis, are located at the back of the ATP-binding domain, whereas Hda is thought to interact with the ATP-binding pocket itself. The intro- duction into B. subtilis of a dnaAY144C mutation, which disabled the interaction with YabA but did not affect interactions either with DnaA itself or with DnaD, resulted in over-initiation and asynchronous initiation of replication and disabled the formation of YabA foci, further demonstrating that the amino acid on the oppo- site side to the ATP-binding pocket is important for YabA binding. -

Changing Perspectives on the Role of Dnaa-ATP in Orisome Function and Timing Regulation
fmicb-10-02009 August 28, 2019 Time: 17:19 # 1 REVIEW published: 29 August 2019 doi: 10.3389/fmicb.2019.02009 Changing Perspectives on the Role of DnaA-ATP in Orisome Function and Timing Regulation Alan C. Leonard1*, Prassanna Rao2, Rohit P. Kadam1 and Julia E. Grimwade1 1 Laboratory of Microbial Genetics, Department of Biomedical and Chemical Engineering and Science, Florida Institute of Technology, Melbourne, FL, United States, 2 Department of Biochemistry, Vanderbilt University School of Medicine, Nashville, TN, United States Bacteria, like all cells, must precisely duplicate their genomes before they divide. Regulation of this critical process focuses on forming a pre-replicative nucleoprotein complex, termed the orisome. Orisomes perform two essential mechanical tasks that configure the unique chromosomal replication origin, oriC to start a new round of chromosome replication: (1) unwinding origin DNA and (2) assisting with loading of the replicative DNA helicase on exposed single strands. In Escherichia coli, a necessary orisome component is the ATP-bound form of the bacterial initiator protein, DnaA. DnaA- ATP differs from DnaA-ADP in its ability to oligomerize into helical filaments, and in its ability to access a subset of low affinity recognition sites in the E. coli replication origin. Edited by: The helical filaments have been proposed to play a role in both of the key mechanical Ludmila Chistoserdova, tasks, but recent studies raise new questions about whether they are mandatory for University of Washington, allADP United States orisome activity. It was recently shown that a version of E. coli oriC (oriC ), whose Reviewed by: multiple low affinity DnaA recognition sites bind DnaA-ATP and DnaA-ADP similarly, was Anders Løbner-Olesen, fully occupied and unwound by DnaA-ADP in vitro, and in vivo suppressed the lethality University of Copenhagen, Denmark of DnaA mutants defective in ATP binding and ATP-specific oligomerization.