Oligomeric Nucleic Acids As Antivirals
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sequence-Selective Recognition of Double-Stranded RNA And
Downloaded from rnajournal.cshlp.org on October 8, 2021 - Published by Cold Spring Harbor Laboratory Press Hnedzko et al. Sequence-Selective Recognition of Double-Stranded RNA and Enhanced Cellular Uptake of Cationic Nucleobase and Backbone- Modified Peptide Nucleic Acids Dziyana Hnedzko1,*, Dennis W. McGee,2 Yannis A. Karamitas1 and Eriks Rozners1,* Departments of 1 Chemistry and 2 Biological Sciences, Binghamton University, The State University of New York, Binghamton, New York 13902, United States. * To whom correspondence should be addressed. Tel: +1-607-777-2441; Fax: +1-607-777- 4478; Email: [email protected] and [email protected] Running Tittle: RNA recognition and cellular uptake of PNA 1 Downloaded from rnajournal.cshlp.org on October 8, 2021 - Published by Cold Spring Harbor Laboratory Press Hnedzko et al. ABSTRACT Sequence-selective recognition of complex RNAs in live cells could find broad applications in biology, biomedical research and biotechnology. However, specific recognition of structured RNA is challenging and generally applicable and effective methods are lacking. Recently, we found that peptide nucleic acids (PNAs) were unusually well suited ligands for recognition of double-stranded RNAs. Herein, we report that 2-aminopyridine (M) modified PNAs and their conjugates with lysine and arginine tripeptides form strong (Ka = 9.4 to 17 × 107 M-1) and sequence-selective triple helices with RNA hairpins at physiological pH and salt concentration. The affinity of PNA-peptide conjugates for the matched RNA hairpins was unusually high compared to the much lower affinity for DNA hairpins of the same 7 -1 sequence (Ka = 0.05 to 0.11 × 10 M ). The binding of double-stranded RNA by M-modified 4 -1 -1 PNA-peptide conjugates was a relatively fast process (kon = 2.9 × 10 M s ) compared to 3 -1 -1 the notoriously slow triple helix formation by oligodeoxynucleotides (kon ~ 10 M s ). -

Curr.Opin.Chem. Biol., 15, 587-594
Available online at www.sciencedirect.com Bacterial replicases and related polymerases Charles S McHenry Bacterial replicases are complex, tripartite replicative replication that are conserved among all life forms are machines. They contain a polymerase, Pol III, a b2 processivity illustrated in Figure 1. factor and a DnaX complex ATPase that loads b2 onto DNA and chaperones Pol III onto the newly loaded b2. Many Structure and function of a, the catalytic bacteria encode both a full length t and a shorter g form of subunit DnaX by a variety of mechanisms. The polymerase catalytic Like all polymerases, Pol III a contains palm, thumb and subunit of Pol III, a, contains a PHP domain that not only binds fingers domains, in the shape of a cupped right hand 2+ to prototypical e Mg -dependent exonuclease, but also Figure 2. However, apo-enzyme structures of the full 2+ contains a second Zn -dependent proofreading length Thermus aquaticus (Taq) and a truncated version of exonuclease, at least in some bacteria. Replication of the E. coli (Eco) a subunit revealed a big surprise: the palm chromosomes of low GC Gram-positive bacteria require two domain has the basic fold of the X family of DNA Pol IIIs, one of which, DnaE, appears to extend RNA primers a polymerases, which includes the slow, non-processive only short distance before handing the product off to the major Pol bs [1 ,2 ]. replicase, PolC. Other bacteria encode a second Pol III (ImuC) that apparently replaces Pol V, required for induced A ternary complex of a dideoxy-terminated primer-tem- mutagenesis in E. -

Nuclear and Mitochondrial DNA Sequences from Two Denisovan Individuals
Nuclear and mitochondrial DNA sequences from two Denisovan individuals Susanna Sawyera,1, Gabriel Renauda,1, Bence Violab,c,d, Jean-Jacques Hublinc, Marie-Theres Gansaugea, Michael V. Shunkovd,e, Anatoly P. Dereviankod,f, Kay Prüfera, Janet Kelsoa, and Svante Pääboa,2 aDepartment of Evolutionary Genetics, Max Planck Institute for Evolutionary Anthropology, D-04103 Leipzig, Germany; bDepartment of Anthropology, University of Toronto, Toronto, ON M5S 2S2, Canada; cDepartment of Human Evolution, Max Planck Institute for Evolutionary Anthropology, D-04103 Leipzig, Germany; dInstitute of Archaeology and Ethnography, Russian Academy of Sciences, Novosibirsk, RU-630090, Russia; eNovosibirsk National Research State University, Novosibirsk, RU-630090, Russia; and fAltai State University, Barnaul, RU-656049, Russia Contributed by Svante Pääbo, October 13, 2015 (sent for review April 16, 2015; reviewed by Hendrik N. Poinar, Fred H. Smith, and Chris B. Stringer) Denisovans, a sister group of Neandertals, have been described on DNA to the ancestors of present-day populations across Asia the basis of a nuclear genome sequence from a finger phalanx and Oceania suggests that in addition to the Altai Mountains, (Denisova 3) found in Denisova Cave in the Altai Mountains. The they may have lived in other parts of Asia. In addition to the only other Denisovan specimen described to date is a molar (Deni- finger phalanx, a molar (Denisova 4) was found in the cave in sova 4) found at the same site. This tooth carries a mtDNA se- 2000. Although less than 0.2% of the DNA in the tooth derives quence similar to that of Denisova 3. Here we present nuclear from a hominin source, the mtDNA was sequenced and differed DNA sequences from Denisova 4 and a morphological description, from the finger phalanx mtDNA at only two positions, suggesting as well as mitochondrial and nuclear DNA sequence data, from it too may be from a Denisovan (2, 3). -

Hammerhead Ribozymes Against Virus and Viroid Rnas
Hammerhead Ribozymes Against Virus and Viroid RNAs Alberto Carbonell, Ricardo Flores, and Selma Gago Contents 1 A Historical Overview: Hammerhead Ribozymes in Their Natural Context ................................................................... 412 2 Manipulating Cis-Acting Hammerheads to Act in Trans ................................. 414 3 A Critical Issue: Colocalization of Ribozyme and Substrate . .. .. ... .. .. .. .. .. ... .. .. .. .. 416 4 An Unanticipated Participant: Interactions Between Peripheral Loops of Natural Hammerheads Greatly Increase Their Self-Cleavage Activity ........................... 417 5 A New Generation of Trans-Acting Hammerheads Operating In Vitro and In Vivo at Physiological Concentrations of Magnesium . ...... 419 6 Trans-Cleavage In Vitro of Short RNA Substrates by Discontinuous and Extended Hammerheads ........................................... 420 7 Trans-Cleavage In Vitro of a Highly Structured RNA by Discontinuous and Extended Hammerheads ........................................... 421 8 Trans-Cleavage In Vivo of a Viroid RNA by an Extended PLMVd-Derived Hammerhead ........................................... 422 9 Concluding Remarks and Outlooks ........................................................ 424 References ....................................................................................... 425 Abstract The hammerhead ribozyme, a small catalytic motif that promotes self- cleavage of the RNAs in which it is found naturally embedded, can be manipulated to recognize and cleave specifically -

Ribozymes Targeted to the Mitochondria Using the 5S Ribosomal Rna
RIBOZYMES TARGETED TO THE MITOCHONDRIA USING THE 5S RIBOSOMAL RNA By JENNIFER ANN BONGORNO A DISSERTATION PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY UNIVERSITY OF FLORIDA 2005 Copyright 2005 by Jennifer Bongorno To my grandmother, Hazel Traster Miller, whose interest in genealogy sparked my interest in genetics, and without whose mitochondria I would not be here ACKNOWLEDGMENTS I would like to thank all the members of the Lewin lab; especially my mentor, Al Lewin. Al was always there for me with suggestions and keeping me motivated. He and the other members of the lab were like my second family; I would not have had an enjoyable experience without them. Diana Levinson and Elizabeth Bongorno worked with me on the fourth and third mouse transfections respectively. Joe Hartwich and Al Lewin tested some of the ribozymes in vitro and cloned some of the constructs I used. James Thomas also helped with cloning and was an invaluable lab manager. Verline Justilien worked on a related project and was a productive person with whom to bounce ideas back and forth. Lourdes Andino taught me how to use the new phosphorimager for my SYBR Green-stained gels. Alan White was there through it all, like the older brother I never had. Mary Ann Checkley was with me even longer than Alan, since we both came to Florida from Ohio Wesleyan, although she did manage to graduate before me. Jia Liu and Frederic Manfredsson were there when I needed a beer. -
Supplementary Data TMAO Microbiome R1
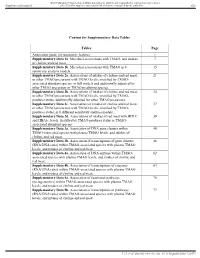
BMJ Publishing Group Limited (BMJ) disclaims all liability and responsibility arising from any reliance Supplemental material placed on this supplemental material which has been supplied by the author(s) Gut Content for Supplementary Data Tables Tables Page Annotation guide for taxonomic features. 1 Supplementary Data 1a. Microbial associations with TMAO, and intakes 7 of choline and red meat. Supplementary Data 1b. Microbial associations with TMAO in 8 15 sensitivity analysis models. Supplementary Data 2a. Associations of intakes of choline and red meat, 21 or other TMAO precursors with TMAO levels, stratified by TMAO- associated abundant species, in full models and additionally adjusted for other TMAO precursors or TMAO predicting species. Supplementary Data 2b. Associations of intakes of choline and red meat, 34 or other TMAO precursors with TMAO levels, stratified by TMAO- producer status, additionally adjusted for other TMAO precursors. Supplementary Data 2c. Associations of intakes of choline and red meat, 37 or other TMAO precursors with TMAO levels, stratified by TMAO- producer status, in 6 different sensitivity analysis models. Supplementary Data 2d. Associations of intakes of red meat with HDLC 39 and HBA1c levels, stratified by TMAO-producer status or TMAO- associated abundant species. Supplementary Data 3a. Association of DNA gene clusters within 40 TMAO-associated species with plasma TMAO levels, and intakes of choline and red meat. Supplementary Data 3b. Association of transcriptions of gene clusters 46 (RNA/DNA ratio) within TMAO-associated species with plasma TMAO levels, and intakes of choline and red meat. Supplementary Data 4a. Association of DNA enzyme within TMAO- 62 associated species with plasma TMAO levels, and intakes of choline and red meat. -

Into the Mitochondrial and Nuclear DNA and RNA of Two Transplantable Hepatomas (3924A and H-35Tc2) and Host Livers'
[CANCERRESEARCH28.2164-2167,October1968] Brief Communication Comparative Incorporation of Tritiated Thymidine and Cytidine into the Mitochondrial and Nuclear DNA and RNA of Two Transplantable Hepatomas (3924A and H-35tc2) and Host Livers' Lillie 0. Chang, Harold P.Morris,2and William B. Looney Radiobiology and Biophysics Laboratory, Department of Radiology, University of Virginia School of Medicine, Charlottesville, Vir ginia f@29O1(L. 0. C. and W. B. L.), and Laboratory of Biochemistry, National Cancer institute, NIH, Bethesda, Maryland ?LXJ114 (H. P. M.) Summary interesting questions as to the different precursor pools and It has been found that the specific activity of thymidine-5- the biosynthetic pathways of nucleic acid synthesis in the methyl-3H incorporation into the mitochondrial DNA of two mitochondria and nuclei of the hepatomas as well as of their transplantable hepatomas (3924A and H-35tc2) and host livers host livers. The purpose of this communication is to report the is less than the specific activity of the nuclear DNA. However, relative rates of uptake of the labeled thymidine and cytidine the ratio of mitochondrial DNA to nuclear DNA specific ac into DNA, and the incorporation of cytidine into RNA of the tivity in both hepatomas and host livers is 10—100times greater mitochondrial and nuclear fractions of Hepatomas 3924A and H-35tc2 and their host livers. following cytidine-5-3H administration than the ratio following thymidine-5-methyl-3H administration. This marked difference in the ratios of the specific activities of thymidine and cytidine Materials and Methods in mitochondrial and nuclear DNA of hepatomas and host livers The growth patterns of transplantable rat hepatoma lines appears to be the result of both an increase in cytidine incor have been described by Morris (8, 9) . -

Isolation of Active Genes Containing CAG Repeats by DNA Strand Invasion by a Peptide Nucleic Acid (Chromatin/Transcription Factors/Microsatellite DNA) LIDIA C
Proc. Natl. Acad. Sci. USA Vol. 92, pp. 1901-1905, March 1995 Biochemistry Isolation of active genes containing CAG repeats by DNA strand invasion by a peptide nucleic acid (chromatin/transcription factors/microsatellite DNA) LIDIA C. BOFFA*, ELISABETrA M. CARPANETO*, AND VINCENT G. ALLFREYtt *Istituto Nazionale per la Ricerca sul Cancro IST, Genoa 16132, Italy; and tThe Rockefeller University, New York, NY 10021 Communicated by Bruce Merrifield, The Rockefeller University, New York, NY December 7, 1994 ABSTRACT An amplification of tandem CAG trinucle- can be captured quantitatively on streptavidin-coated mag- otide sequences in DNA due to errors in DNA replication is netic beads. involved in at least four hereditary neurodegenerative dis- An amplification of CAG triplet repeats occurs in at least eases. The CAG triplet repeats when translated into protein four inherited neurodegenerative diseases. In all cases, the give rise to tracts of glutamine residues, which are a promi- severity of the disease increases with the expansion of the CAG nent feature of many transcription factors, including the repeat region that occurs as a consequence of errors in DNA TATA-binding protein of transcription factor TFIID. We have replication. In Huntington disease, expansion of the CAG used a biotin-labeled, complementary peptide nucleic acid repeats in the gene results in an elongation of a glutamine-rich (PNA) to invade the CAG repeats in intact chromatin and then domain in the encoded protein from 35 to over 100 residues employed a method for the selective isolation of transcrip- (11). The functional consequence of similar glutamine expan- tionally active chromatin restriction fragments containing the sions for transcriptional control is indicated by the finding that PNA-DNA hybrids. -

Non-Coding RNA: Microrna Stimulates Mitochondrial Translation
RESEARCH HIGHLIGHTS Nature Reviews Genetics | AOP, published online 12 August 2014 IN BRIEF THERAPEUTICS Targeting huntingtin through morpholino oligomers Huntington’s disease is caused by a mutant huntingtin (HTT) gene that contains an expanded tract of poly(CAG) repeats. Sun et al. designed phosphorodiamidate morpholino oligomers (PMOs, which are stable nucleic acid mimics) as antisense reagents to target the CAG tract in HTT mRNA. Applying the PMOs to HTT-mutant human neurons in vitro decreased HTT protein levels and reduced toxicity caused by mutant HTT. Furthermore, in two mouse models of Huntington’s disease, intracranial injection of PMOs resulted in downregulation of mutant HTT levels and partially reduced disease symptoms. ORIGINAL RESEARCH PAPER Sun, X. et al. Phosphorodiamidate morpholino oligomers suppress mutant huntingtin expression and attenuate neurotoxicity. Hum. Mol. Genet. http://dx.doi.org/10.1093/hmg/ddu349 (2014) TECHNOLOGY Using DNA repair to detect modified bases There is great interest in characterizing the locations and functions of chemically modified bases in genomes. Bryan et al. report their Excision-seq method, in which DNA repair enzymes are used to cut genomic DNA at sites of the particular damaged bases they recognize, followed by high-throughput sequencing to characterize these cleavage sites. The researchers characterized the locations and sequence contexts of uracils (that is, demethylated thymines) in Escherichia coli and Saccharomyces cerevisiae genomes, and of ultraviolet-light-induced pyrimidine dimers in S. cerevisiae. ORIGINAL RESEARCH PAPER Bryan, D. S. et al. High resolution mapping of modified DNA nucleobases using excision repair enzymes. Genome Res. http://dx.doi.org/10.1101/ gr.174052.114 (2014) NON-CODING RNA MicroRNA stimulates mitochondrial translation The muscle-specific microRNA miR‑1 stimulates translation of various transcripts encoded by mitochondrial DNA, while repressing its nuclear DNA-encoded targets in the cytoplasm, report Zhang and colleagues. -

Ordered Association of Helicase Loader Proteins with the Bacillus Subtilis Origin of Replication in Vivo
Ordered association of helicase loader proteins with the Bacillus subtilis origin of replication in vivo The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation Smits, Wiep Klaas, Alexi I. Goranov, and Alan D. Grossman. “Ordered association of helicase loader proteins with the Bacillus subtilis origin of replication in vivo.” Molecular Microbiology 75.2 (2010): 452–461. As Published http://dx.doi.org/10.1111/j.1365-2958.2009.06999.x Publisher Wiley Blackwell (Blackwell Publishing) Version Author's final manuscript Citable link http://hdl.handle.net/1721.1/73616 Terms of Use Creative Commons Attribution-Noncommercial-Share Alike 3.0 Detailed Terms http://creativecommons.org/licenses/by-nc-sa/3.0/ NIH Public Access Author Manuscript Mol Microbiol. Author manuscript; available in PMC 2011 January 1. NIH-PA Author ManuscriptPublished NIH-PA Author Manuscript in final edited NIH-PA Author Manuscript form as: Mol Microbiol. 2010 January ; 75(2): 452±461. doi:10.1111/j.1365-2958.2009.06999.x. Ordered association of helicase loader proteins with the Bacillus subtilis origin of replication in vivo Wiep Klaas Smits, Alexi I. Goranov, and Alan D. Grossman* Department of Biology, Massachusetts Institute of Technology, Cambridge, MA 02139 Summary The essential proteins DnaB, DnaD, and DnaI of Bacillus subtilis are required for initiation, but not elongation, of DNA replication, and for replication restart at stalled forks. The interactions and functions of these proteins have largely been determined in vitro based on their roles in replication restart. During replication initiation in vivo, it is not known if these proteins, and the replication initiator DnaA, associate with oriC independently of each other by virtue of their DNA binding activities, as a (sub)complex like other loader proteins, or in a particular dependent order. -

A Free-Living Protist That Lacks Canonical Eukaryotic DNA Replication and Segregation Systems
bioRxiv preprint doi: https://doi.org/10.1101/2021.03.14.435266; this version posted March 15, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 A free-living protist that lacks canonical eukaryotic DNA replication and segregation systems 2 Dayana E. Salas-Leiva1, Eelco C. Tromer2,3, Bruce A. Curtis1, Jon Jerlström-Hultqvist1, Martin 3 Kolisko4, Zhenzhen Yi5, Joan S. Salas-Leiva6, Lucie Gallot-Lavallée1, Geert J. P. L. Kops3, John M. 4 Archibald1, Alastair G. B. Simpson7 and Andrew J. Roger1* 5 1Centre for Comparative Genomics and Evolutionary Bioinformatics (CGEB), Department of 6 Biochemistry and Molecular Biology, Dalhousie University, Halifax, NS, Canada, B3H 4R2 2 7 Department of Biochemistry, University of Cambridge, Cambridge, United Kingdom 8 3Oncode Institute, Hubrecht Institute – KNAW (Royal Netherlands Academy of Arts and Sciences) 9 and University Medical Centre Utrecht, Utrecht, The Netherlands 10 4Institute of Parasitology Biology Centre, Czech Acad. Sci, České Budějovice, Czech Republic 11 5Guangzhou Key Laboratory of Subtropical Biodiversity and Biomonitoring, School of Life Science, 12 South China Normal University, Guangzhou 510631, China 13 6CONACyT-Centro de Investigación en Materiales Avanzados, Departamento de medio ambiente y 14 energía, Miguel de Cervantes 120, Complejo Industrial Chihuahua, 31136 Chihuahua, Chih., México 15 7Centre for Comparative Genomics and Evolutionary Bioinformatics (CGEB), Department of 16 Biology, Dalhousie University, Halifax, NS, Canada, B3H 4R2 17 *corresponding author: [email protected] 18 D.E.S-L ORCID iD: 0000-0003-2356-3351 19 E.C.T. -

Product Sheet Info
Master Clone List for NR-19278 Streptococcus pneumoniae, Strain TIGR4, Gateway® Clone Set, Recombinant in Escherichia coli, Plates 1-23 Catalog No. NR-19278 Table 1: Streptococcus pneumoniae, Strain TIGR4, Gateway® Clone, Plate 1 (YSPCA), NR-195681 Well ORF Accession Average Depth of Clone Locus ID Description Position Length Number Coverage 22040 A01 SP1028 hypothetical protein SP_1028 984 NP_345503.1 8.94410569 22062 A02 SP0684 hypothetical protein SP_0684 681 NP_345189.1 5.09985316 22066 A03 SP0747 hypothetical protein SP_0747 729 NP_345246.1 12.4828532 22067 A04 SP0934 hypothetical protein SP_0934 888 NP_345418.1 8.29617117 22068 A05 SP0612 hypothetical protein SP_0612 627 NP_345124.1 3.25039872 22074 A06 SP1678 hypothetical protein SP_1678 838 NP_346117.1 4.81622912 22075 A07 SP0699 hypothetical protein SP_0699 690 NP_345203.1 12.3768116 22076 A08 SP0195 hypothetical protein SP_0194 294 NP_344735.1 4.7755102 22080 A09 SP0821 hypothetical protein SP_0821 795 NP_345313.1 9.35345912 22081 A10 SP0407 hypothetical protein SP_0407 471 NP_344930.1 4.30997877 22083 A11 SP0070 hypothetical protein SP_0070 195 NP_344619.1 1.95384615 22086 A12 SP0654 hypothetical protein SP_0654 651 NP_345159.1 4.78341014 22089 B01 SP1120 hypothetical protein SP_1120 1104 NP_345591.1 10.7336957 22090 B02 SP0414 hypothetical protein SP_0414 474 NP_344937.1 4.80379747 22091 B03 SP1788 hypothetical protein SP_1788 169 NP_346221.1 1.6035503 22094 B04 SP0172 hypothetical protein SP_0172 270 NP_344713.1 14.9444444 22098 B05 SP0316 hypothetical protein SP_0316 381