The First Data on the Innervation of The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Zootaxa, Mollusca, Goniodorididae, Okenia
ZOOTAXA 695 Further species of the opisthobranch genus Okenia (Nudibranchia: Goniodorididae) from the Indo-West Pacific W.B. RUDMAN Magnolia Press Auckland, New Zealand W.B. RUDMAN Further species of the opisthobranch genus Okenia (Nudibranchia: Goniodorididae) from the Indo-West Pacific (Zootaxa 695) 70 pp.; 30 cm. 25 October 2004 ISBN 1-877354-68-6 (Paperback) ISBN 1-877354-69-4 (Online edition) FIRST PUBLISHED IN 2004 BY Magnolia Press P.O. Box 41383 Auckland 1030 New Zealand e-mail: [email protected] http://www.mapress.com/zootaxa/ © 2004 Magnolia Press All rights reserved. No part of this publication may be reproduced, stored, transmitted or disseminated, in any form, or by any means, without prior written permission from the publisher, to whom all requests to reproduce copyright material should be directed in writing. This authorization does not extend to any other kind of copying, by any means, in any form, and for any purpose other than private research use. ISSN 1175-5326 (Print edition) ISSN 1175-5334 (Online edition) Zootaxa 695: 1–70 (2004) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ ZOOTAXA 695 Copyright © 2004 Magnolia Press ISSN 1175-5334 (online edition) Further species of the opisthobranch genus Okenia (Nudibranchia: Goniodorididae) from the Indo-West Pacific W.B. RUDMAN The Australian Museum, 6 College St., Sydney, NSW 2010, Australia. E-mail: [email protected]. Table of Contents Abstract . 3 Introduction. 4 Materials and Methods . 5 Descriptions . 5 Okenia Menke, 1830. 5 Okenia echinata Baba, 1949. 6 Okenia purpurata sp. nov. 9 Okenia vena sp. nov.. 13 Okenia virginiae Gosliner, 2004. -

Review Sheet for Lecture 15: Life in the Benthic Realm
Lecture 15: Life in the Benthic Realm Terminology Planktonic, nektonic, benthic, epifaunal, infaunal, sessile, mobile, bathyal, abyssal, abyssopelagic, hadopelagic, supratidal, intertidal, subtidal, spicules, spongin, radial symmetry, encruster, epidermal cell, porocyte, sclerocyte, amoebocyte, mesohyl, choanocyte, polyp, medusa, colonial, solitary, aragonite, octocoral, gorgonin, cnidocyst, nematocyst, zooxanthellae, hematypic, coral bleaching, lophophore, zoid, zooecium, zooaria, polymorphism, pedicle, helically coiled shell, radially coiled shell, exoskeleton, endoskeleton Dates: none Review Questions: Be familiar with the life and feeding modes of all of the invertebrate groups we discussed and their major morphologies, behaviors, life strategies etc. Why are so many sessile invertebrates radially symmetric? Explain the three different wall types found in sponges, what’s the point? Discuss the different roles of the different sponge cells How do hematypic corals contribute to the creation of different nearshore environments and conditions? Describe the community created by an innkeeper worm How does the skeleton of a brachiopod differ from that of a clam? How do the shells of clams, snails, and nautiloids differ? Classification: Phylum Porifera Class Demospongiae (soft sponges) Class Calcarea (calcareous sponges) Class Hexactinellida (glass sponges) Phylum Cnidaria Class Anthozoa -stony corals -sea fans -soft corals -sea anemones -sea pens Phylum Annelida (worms) -bristleworms -innkeeper worm -lugworms -spaghetti worm -feather duster -catworm Phylum Bryozoa (moss-animals) Phylum Mollusca -Class Bivalvia (clams) -Class Gastropoda (snails and slugs) -Class Cephalopoda (squid, octopus, nautiloid) -Class Scaphopoda (tusk shells) -Class Polyplacophora (chitons) . -
Bryozoa of the Caspian Sea
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/339363855 Bryozoa of the Caspian Sea Article in Inland Water Biology · January 2020 DOI: 10.1134/S199508292001006X CITATIONS READS 0 90 1 author: Valentina Ivanovna Gontar Russian Academy of Sciences 58 PUBLICATIONS 101 CITATIONS SEE PROFILE Some of the authors of this publication are also working on these related projects: Freshwater Bryozoa View project Evolution of spreading of marine invertebrates in the Northern Hemisphere View project All content following this page was uploaded by Valentina Ivanovna Gontar on 19 February 2020. The user has requested enhancement of the downloaded file. ISSN 1995-0829, Inland Water Biology, 2020, Vol. 13, No. 1, pp. 1–13. © Pleiades Publishing, Ltd., 2020. Russian Text © The Author(s), 2020, published in Biologiya Vnutrennykh Vod, 2020, No. 1, pp. 3–16. AQUATIC FLORA AND FAUNA Bryozoa of the Caspian Sea V. I. Gontar* Institute of Zoology, Russian Academy of Sciences, St. Petersburg, Russia *e-mail: [email protected] Received April 24, 2017; revised September 18, 2018; accepted November 27, 2018 Abstract—Five bryozoan species of the class Gymnolaemata and a single Plumatella emarginata species of the class Phylactolaemata are found in the Caspian Sea. The class Gymnolaemata is represented by bryozoans of the orders Ctenostomatida (Amathia caspia, Paludicella articulata, and Victorella pavida) and Cheilostoma- tida (Conopeum grimmi and Lapidosella ostroumovi). Two species (Conopeum grimmi and Amatia caspia) are Caspian endemics. Lapidosella ostroumovi was identified in the Caspian Sea for the first time. The systematic position, illustrated morphological descriptions, and features of ecology of the species identified are pre- sented. -

Invertebrates Invertebrates: • Are Animals Without Backbones • Represent 95% of the Animal Kingdom Animal Diversity Morphological Vs
Invertebrates Invertebrates: • Are animals without backbones • Represent 95% of the animal kingdom Animal Diversity Morphological vs. Molecular Character Phylogeny? A tree is a hypothesis supported or not supported by evidence. Groupings change as new evidence become available. Sponges - Porifera Natural Bath Sponges – over-collected, now uncommon Sponges • Perhaps oldest animal phylum (Ctenphora possibly older) • may represent several old phyla, some now extinct ----------------Ctenophora? Sponges - Porifera • Mostly marine • Sessile animals • Lack true tissues; • Have only a few cell types, cells kind of independent • Most have no symmetry • Body resembles a sac perforated with holes, system of canals. • Strengthened by fibers of spongin, spicules Sponges have a variety of shapes Sponges Pores Choanocyte Amoebocyte (feeding cell) Skeletal Water fiber flow Central cavity Flagella Choanocyte in contact with an amoebocyte Sponges - Porifera • Sessile filter feeder • No mouth • Sac-like body, perforated by pores. • Interior lined by flagellated cells (choanocytes). Flagellated collar cells generate a current, draw water through the walls of the sponge where food is collected. • Amoeboid cells move around in the mesophyll and distribute food. Sponges - Porifera Grantia x.s. Sponge Reproduction Asexual reproduction • Fragmentation or by budding. • Sponges are capable of regeneration, growth of a whole from a small part. Sexual reproduction • Hermaphrodites, produce both eggs and sperm • Eggs and sperm released into the central cavity • Produces -
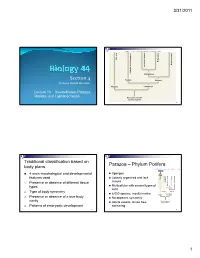
S I Section 4
3/31/2011 Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Porifera Ecdysozoa Deuterostomia Lophotrochozoa Cnidaria and Ctenophora Cnidaria and Protostomia SSiection 4 Radiata Bilateria Professor Donald McFarlane Parazoa Eumetazoa Lecture 13 Invertebrates: Parazoa, Radiata, and Lophotrochozoa Ancestral colonial choanoflagellate 2 Traditional classification based on Parazoa – Phylum Porifera body plans Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Parazoa 4 main morphological and developmental Sponges features used Loosely organized and lack Porifera Ecdysozoa Cnidaria and Ctenophora tissues euterostomia photrochozoa D 1. o Presence or absence of different tissue L Multicellular with several types of types Protostomia cells 2. Type of body symmetry Radiata Bilateria 8,000 species, mostly marine Parazoa Eumetazoa 3. Presence or absence of a true body No apparent symmetry cavity Ancestral colonial Adults sessile, larvae free- choanoflagellate 4. Patterns of embryonic development swimming 3 4 1 3/31/2011 Water drawn through pores (ostia) into spongocoel Flows out through osculum Reproduce Choanocytes line spongocoel Sexually Most hermapppgggphrodites producing eggs and sperm Trap and eat small particles and plankton Gametes are derived from amoebocytes or Mesohyl between choanocytes and choanocytes epithelial cells Asexually Amoebocytes absorb food from choanocytes, Small fragment or bud may detach and form a new digest it, and carry -

Practical Paleoecology GEO 342
Practical Paleoecology GEO 342 Compiled By: Dr. Osama Attia Compiled By Dr. Osama Attia 1 Phylum Brachiopoda Brachiopods or lamp shells Name: Name means "arm" (brachio) + "foot" (pod). Main characteristics: - Bivalved (two shells), each with bilateral symmetry. - The plane of symmetry passes through the center of each shell or valve. - The two valves differ in size and shape in most. Sometimes the larger valve will have an opening near the hinge line through which the pedicle extended in life. - Soft parts include a lophophore consisting of coiled tentacles with cilia. The lophophore circulates water between the two valves, distributing oxygen and flushing out carbon dioxide. Water movements caused by the lophophore also transport food particles toward the mouth. Geologic range: Lower Cambrian to Recent. Very abundant during the Paleozoic. A few species (belonging to only three families) remain today. Mode of life: Inhabitants of shallow marine environments; they generally live attached in a fixed position on the seafloor. Inarticulate brachiopods are known to live in burrows in the sediment. Brachiopods are filter feeders. Compiled By Dr. Osama Attia 2 Living positions of brachiopods. A = Articulate brachiopod attached to the seafloor by its pedicle. B = Interior of brachiopod valve showing lophophore. C = Inarticulate brachiopod, Lingula, which lives within a tube or burrow in seafloor sediment. A. CLASS INARTICULATA – The Inarticulate Brachiopods Primitive brachiopods with phosphatic or chitinous valves; no hinge. Spoon-shaped valves held together with muscles and soft parts. Lingula is a well known inarticulate brachiopod. Geologic range: Lower Cambrian to Recent Compiled By Dr. Osama Attia 3 Fossil inarticulate brachiopod from the Cambrian. -

BRIOZOÁRIOS DO FOULING: Avaliação Das Espécies Exóticas E Invasoras Do Brasil
UNIVERSIDADE FEDERAL DE PERNAMBUCO CENTRO DE BIOCIÊNCIAS DEPARTAMENTO DE ZOOLOGIA PROGRAMA DE PÓS-GRADUAÇÃO EM BIOLOGIA ANIMAL ADÉLIA ALLIZ MIRANDA BRIOZOÁRIOS DO FOULING: avaliação das espécies exóticas e invasoras do Brasil RECIFE 2018 ADÉLIA ALLIZ MIRANDA BRIOZOÁRIOS DO FOULING: avaliação das espécies exóticas e invasoras do Brasil Dissertação apresentada ao Programa de Pós-Graduação em Biologia Animal da Universidade Federal de Pernambuco como requisito parcial para obtenção do título de Mestre em Biologia Animal. Orientador: Prof. Dr. Leandro Manzoni Vieira RECIFE 2018 Catalogação na fonte: Bibliotecário: Bruno Márcio Gouveia - CRB-4/1788 Miranda, Adélia Alliz Briozoários do fouling : avaliação das espécies exóticas invasoras do Brasil / Adélia Alliz Miranda. – 2018. 121 f. : il. Orientador: Prof. Dr. Leandro Manzoni Vieira. Dissertação (mestrado) – Universidade Federal de Pernambuco. Centro de Biociências. Programa de Pós-graduação em Biologia Animal, Recife, 2018. Inclui referências e apêndices. 1. Animais marinhos. 2. Invertebrados marinhos. I. Vieira, Leandro Manzoni (Orientador). II. Título. 592 CDD (22.ed.) UFPE/CB – 2018 - 373 1 ADÉLIA ALLIZ MIRANDA BRIOZOÁRIOS DO FOULING: avaliação das espécies exóticas e invasoras do Brasil Dissertação apresentada ao Programa de Pós- Graduação em Biologia Animal da Universidade Federal de Pernambuco como requisito parcial para obtenção do título de Mestre em Biologia Animal. Aprovada em: 19/07/2018 BANCA EXAMINADORA _______________________________________________________________ Prof. Dr. André Morgado Esteves Universidade Federal de Pernambuco ______________________________________________________________ Prof. Dr. Rosana Moreira da Rocha Universidade Federal do Paraná ______________________________________________________ Prof. Dr. Alvaro Esteves Migotto Universidade de São Paulo 2 AGRADECIMENTOS Agradeço em primeiro lugar aos meus pais, Thereza e Antônio, pois tudo que sou devo a eles. -

Animal Diversity Part 2
Textbook resources • pp. 517-522 • pp. 527-8 Animal Diversity • p. 530 part 2 • pp. 531-2 Clicker question In protostomes A. The blastopore becomes the mouth. B. The blastopore becomes the anus. C. Development involves indeterminate cleavage. D. B and C Fig. 25.2 Phylogeny to know (1). Symmetry Critical innovations to insert: Oral bilateral symmetry ecdysis mouth develops after anus multicellularity Aboral tissues 1 Animal diversity, part 2 Parazoa Diversity 2 I. Parazoa • Porifera: Sponges II. Cnidaria & Ctenophora • Tissues • Symmetry I. Outline the • Germ Layers III. Lophotrochozoa unique • Embryonic characteristics Development of sponges IV. Ecdysozoa • Body Cavities • Segmentation Parazoa Parazoa • Porifera: Sponges • Porifera: Sponges – Multicellular without – Hermaphrodites tissues – Sexual and asexual reproduction – Choanocytes (collar cells) use flagella to move water and nutrients into pores – Intracellular digestion Fig. 25.11 Animal diversity, part 2 Clicker Question Diversity 2 I. Parazoa In diploblastic animals, the inner lining of the digestive cavity or tract is derived from II. Cnidaria & Ctenophora A. Endoderm. II. Outline the B. Ectoderm. unique III. Lophotrochozoa C. Mesoderm. characteristics D. Coelom. of cnidarians and IV. Ecdysozoa ctenophores 2 Coral Box jelly Cnidaria and Ctenophora • Cnidarians – Coral; sea anemone; jellyfish; hydra; box jellies • Ctenophores – Comb jellies Sea anemone Jellyfish Hydra Comb jelly Cnidaria and Ctenophora Fig. 25.12 Coral Box jelly Cnidaria and Ctenophora • Tissues Fig. 25.12 – -

First Record of the Non-Native Bryozoan Amathia (= Zoobotryon) Verticillata (Delle Chiaje, 1822) (Ctenostomata) in the Galápagos Islands
BioInvasions Records (2015) Volume 4, Issue 4: 255–260 Open Access doi: http://dx.doi.org/10.3391/bir.2015.4.4.04 © 2015 The Author(s). Journal compilation © 2015 REABIC Rapid Communication First record of the non-native bryozoan Amathia (= Zoobotryon) verticillata (delle Chiaje, 1822) (Ctenostomata) in the Galápagos Islands 1 2,5 3 4 5 6 Linda McCann *, Inti Keith , James T. Carlton , Gregory M. Ruiz , Terence P. Dawson and Ken Collins 1Smithsonian Environmental Research Center, 3152 Paradise Drive, Tiburon, CA 94920, USA 2Charles Darwin Foundation, Marine Science Department, Santa Cruz Island, Galápagos, Ecuador 3Maritime Studies Program, Williams College-Mystic Seaport, 75 Greenmanville Avenue, Mystic, Connecticut 06355, USA 4Smithsonian Environmental Research Center, 647 Contees Wharf Road, Edgewater, MD 21037, USA 5School of the Environment, University of Dundee, Perth Road, Dundee, DD1 4HN, UK 6Ocean and Earth Science, University of Southampton, National Oceanography Centre, Southampton, SO14 3ZH, UK E-mail: [email protected] (LM), [email protected] (IK), [email protected] (JC), [email protected] (GMR), [email protected] (TD), [email protected] (KC) *Corresponding author Received: 9 June 2015 / Accepted: 31 August 2015 / Published online: 18 September 2015 Handling editor: Thomas Therriault Abstract The warm water marine bryozoan Amathia (= Zoobotryon) verticillata (delle Chiaje, 1822) is reported for the first time in the Galápagos Islands based upon collections in 2015. Elsewhere, this species is a major fouling organism that can have significant negative ecological and economic effects. Comprehensive studies will be necessary to determine the extent of the distribution of A. -

Tunicata 4 Alberto Stolfi and Federico D
Tunicata 4 Alberto Stolfi and Federico D. Brown Chapter vignette artwork by Brigitte Baldrian. © Brigitte Baldrian and Andreas Wanninger. A. Stolfi Department of Biology , Center for Developmental Genetics, New York University , New York , NY , USA F. D. Brown (*) EvoDevo Laboratory, Departamento de Zoologia , Instituto de Biociências, Universidade de São Paulo , São Paulo , SP , Brazil Evolutionary Developmental Biology Laboratory, Department of Biological Sciences , Universidad de los Andes , Bogotá , Colombia Centro Nacional de Acuicultura e Investigaciones Marinas (CENAIM) , Escuela Superior Politécnica del Litoral (ESPOL) , San Pedro , Santa Elena , Ecuador e-mail: [email protected] A. Wanninger (ed.), Evolutionary Developmental Biology of Invertebrates 6: Deuterostomia 135 DOI 10.1007/978-3-7091-1856-6_4, © Springer-Verlag Wien 2015 [email protected] 136 A. Stolfi and F.D. Brown Above all , perhaps , I am indebted to a decidedly the phylogenetic relationships between the three vegetative , often beautiful , and generally obscure classes and many orders and families have yet to group of marine animals , both for their intrinsic interest and for the enjoyment I have had in search- be satisfactorily settled. Appendicularia, ing for them . N. J. Berrill (1955) Thaliacea, and Ascidiacea remain broadly used in textbooks and scientifi c literature as the three classes of tunicates; however, recent molecular INTRODUCTION phylogenies have provided support for the mono- phyly of only Appendicularia and Thaliacea, but Tunicates are a group of marine fi lter-feeding not of Ascidiacea (Swalla et al. 2000 ; animals1 that have been traditionally divided into Tsagkogeorga et al. 2009 ; Wada 1998 ). A para- three classes: (1) Appendicularia, also known as phyletic Ascidiacea calls for a reevaluation of larvaceans because their free-swimming and tunicate relationships. -

Nemertean and Phoronid Genomes Reveal Lophotrochozoan Evolution and the Origin of Bilaterian Heads
Nemertean and phoronid genomes reveal lophotrochozoan evolution and the origin of bilaterian heads Author Yi-Jyun Luo, Miyuki Kanda, Ryo Koyanagi, Kanako Hisata, Tadashi Akiyama, Hirotaka Sakamoto, Tatsuya Sakamoto, Noriyuki Satoh journal or Nature Ecology & Evolution publication title volume 2 page range 141-151 year 2017-12-04 Publisher Springer Nature Macmillan Publishers Limited Rights (C) 2017 Macmillan Publishers Limited, part of Springer Nature. Author's flag publisher URL http://id.nii.ac.jp/1394/00000281/ doi: info:doi/10.1038/s41559-017-0389-y Creative Commons Attribution 4.0 International (http://creativecommons.org/licenses/by/4.0/) ARTICLES https://doi.org/10.1038/s41559-017-0389-y Nemertean and phoronid genomes reveal lophotrochozoan evolution and the origin of bilaterian heads Yi-Jyun Luo 1,4*, Miyuki Kanda2, Ryo Koyanagi2, Kanako Hisata1, Tadashi Akiyama3, Hirotaka Sakamoto3, Tatsuya Sakamoto3 and Noriyuki Satoh 1* Nemerteans (ribbon worms) and phoronids (horseshoe worms) are closely related lophotrochozoans—a group of animals including leeches, snails and other invertebrates. Lophotrochozoans represent a superphylum that is crucial to our understand- ing of bilaterian evolution. However, given the inconsistency of molecular and morphological data for these groups, their ori- gins have been unclear. Here, we present draft genomes of the nemertean Notospermus geniculatus and the phoronid Phoronis australis, together with transcriptomes along the adult bodies. Our genome-based phylogenetic analyses place Nemertea sis- ter to the group containing Phoronida and Brachiopoda. We show that lophotrochozoans share many gene families with deu- terostomes, suggesting that these two groups retain a core bilaterian gene repertoire that ecdysozoans (for example, flies and nematodes) and platyzoans (for example, flatworms and rotifers) do not. -
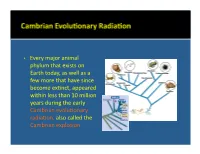
• Every Major Animal Phylum That Exists on Earth Today, As Well As A
• Every major animal phylum that exists on Earth today, as well as a few more that have since become ex:nct, appeared within less than 10 million years during the early Cambrian evolu:onary radiaon, also called the Cambrian explosion. • Phylum Pro:sta includes a diverse group of eukaryo:c microorganisms which are mostly unicellular. They are not necessarily primi:ve, although some are. Only some have a skeleton that can be preserved as a fossil. • Foraminifera and radiolaria are the most important examples. Coccolithophores, diatoms, and dinoflagellates are also examples. • Foraminifera are marine organisms that may be either planktonic, living in the water column where they float at various levels, or benthic, living on or within the seafloor sediment. • They form relavely large (0.1 mm to 5 cm) porous internal skeletons called tests through which project strands of living cytoplasm. The single- to mul:-chambered tests may be composed of organic maer, agglu:nated sand grains or sponge spicules, or calcium carbonate. • Benthic foraminifera • Planktonic foraminifera (Cambrian to Recent). (Jurassic to Recent). Oligocene Eocene • Radiolaria are planktonic marine organisms that tend to live in relavely cold water. • Their tests, oNen delicate and elaborate, are made of silica and presently accumulate on the floors of the parts of oceans that are deeper than those where foraminiferal tests are accumulang. • Radiolaria (Cambrian to • Radiolaria (Cambrian to Recent). Recent). Paleocene Radiolaria • Coccolithophores (Triassic to Recent) are extremely small marine planktonic photosynthe:c unicellular algae that are enclosed by plates of low-Mg calcite called coccoliths. Cretaceous White Cliffs of Dover, England • Diatoms (Jurassic to Recent) are small freshwater and marine planktonic photosynthe:c unicellular algae that are enclosed by a cell wall made of silica called a frustule.