Thyroid Vascularity and Blood Flow
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Thyroid Hormone Misuse and Abuse
Endocrine (2019) 66:79–86 https://doi.org/10.1007/s12020-019-02045-1 REVIEW Thyroid hormone misuse and abuse Victor J. Bernet 1,2 Received: 11 June 2019 / Accepted: 2 August 2019 © Springer Science+Business Media, LLC, part of Springer Nature 2019 Abstract Thyroid hormone (TH) plays an essential role in human physiology and maintenance of appropriate levels is important for good health. Unfortunately, there are instances in which TH is misused or abused. Such misuse may be intentional such as when individuals take thyroid hormone for unapproved indications like stimulation of weight loss or improved energy. There are instances where healthcare providers prescribe thyroid hormone for controversial or out of date uses and sometimes in supraphysiologic doses. Othertimes, unintentional exposure may occur through supplements or food that unknowingly contain TH. No matter the reason, exposure to exogenous forms of TH places the public at risk for potential adverse side effects. Keywords Thyrotoxicosis ● Thyroid hormone abuse ● Thyroid hormone misuse ● Factitious thyrotoxicosis ● Supplements ● 1234567890();,: 1234567890();,: Analogs Overdose Thyroid hormone misuse and abuse secondary to exogenous TH ingestion that may be unin- tentional or purposeful such as in some cases of Munch- Almost any substance that impacts body physiology and ausen syndrome. Instances of TH misuse can be associated function is at risk for misuse or frank abuse, usually in the with the development of significant side effects. There are misgiven belief that the particular agent whether it be a other instances of chronic, low-grade over treatment with herb, supplement, medication, or hormone has healing TH, which occur for various misreasoning such as weight properties beyond that of which it actually possesses. -

A Case of Thyrotoxic Paralysis Caused by Consumption of Iodocaseine
Journal of Health and Social Sciences 2019; 4,2:277-282 CASE REPORT IN EMERGENCY MEDICINE A case of thyrotoxic paralysis caused by consumption of iodocaseine Antonio Villa1, Gabriella Nucera2 Affiliations: 1 M.D., Department of Emergency, ASST Monza, PO Desio, Monza, Italy 2 M.D., Department of Emergency Medicine, ASST Fatebenefratelli-Sacco, PO Fatebenefratelli, Milano, Italy Corresponding author: Dr Antonio Villa, ASST Monza, PO Desio. Via Fiuggi, 56 20159 Milan, Italy. E-mail: [email protected] Abstract Acute hypokalaemic paralysis is a rare but treatable cause of acute limb weakness. Thyrotoxic paralysis is an uncommon, potentially life-threatening endocrine emergency and it is a rare complication of hyperthyroidism. The most common causes of hyperthyroidism include Graves’ disease, multinodular goiters or solitary thyroid nodule, and iodine-induced thyrotoxicosis ( Jod-Basedow syndrome). Thyreotoxicosis factitia, which is caused by the excessive ingestion of exogenous thyroid hormone or iodine derivatives administration, has been rarely reported as a cause of thyrotoxic paralysis. We describe the case of a young Caucasian male with flaccid paralysis of all four limbs and severe hypokalaemia after inappropriate iodine derivatives (iodocasein) intake to show, in conclusion, how critical care physicians need to be aware of this rare but curable condition. KEY WORDS: Iodocaseine; hypokalaemia; thyrotoxic paralysis. 277 Journal of Health and Social Sciences 2019; 4,2:277-282 Riassunto La paralisi acuta ipokaliemica è una causa rara ma curabile di astenia acuta. La paralisi tireotossica è un'e- mergenza endocrina non comune e potenzialmente pericolosa per la vita ed è una rara complicanza dell'i- pertiroidismo. L'ipertiroidismo è causato prevalentemente dalla malattia di Graves, da gozzi singoli o multi- nodulari, e dalla malattia indotta da iodio ( Jod-Basedow). -
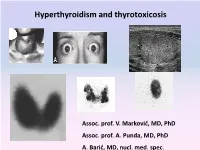
Hyperthyroidism and Thyrotoxicosis
Hyperthyroidism and thyrotoxicosis Assoc. prof. V. Marković, MD, PhD Assoc. prof. A. Punda, MD, PhD A. Barić, MD, nucl. med. spec. Hyperthyroidism- Thyrotoxicosis Hyperthyroidism- elevated serum levels of thyroid hormones caused by overproduction of thyroid hormones Thyrotoxicosis: elevated serum level of thyroid hormones/ excessive amount of circulating thyroid hormone Hyperthyreoidism includes thyreotoxycosis but Thyrotoxicosis is not exclusively caused by hyperthyroidism Classification of thyrotoxicosis Hyperthyroidism Thyrotoxicosis without hyperthyroidism Mb Basedow-Graves Thyrotoxicosis factitia Multinodular toxic goiter Subacute thyroiditis (painfull) Toxic adenoma Subacute thyroiditis (painless) Elevated TSH levels Ectopic thyroid tissue Trophoblastic tumors Iod-Basedow Diffuse toxic goiter (Mb Basedow, Graves) Diffuse toxic goiter Mb. Graves-Basedow Epidemiology and etiology Diffuse toxic goiter (Mb. Graves- Basedow) is an autoimune, multysistemic dissease, wich includes the thyoid gland, infiltrative ophthalmopathy, dermopathy and acropathy. World wide prevalence is about 0,4-2% in women, 0,1% in men, includes 60-90% of hyperthyroidism cases*. It is complex disease with predominant genetic component.& Sex hormones, stress. Disease is caused by TSH receptor stimulating antibodies/ TSH stimulating antibodies (TSAb), in 80-100% patients. *Taunbridge WMG, Vanderpump MPJ. Population screening for autoimmune thyroid disease. Endocriol Metab Clin North Am. 2000;29:239-253. &Brix TH, Kyvik KO, Christensen K, et al. Evdence for a major -

Thyroid Vascularity and Blood Flow
European Journal of Endocrinology (1999) 140 452–456 ISSN 0804-4643 Thyroid vascularity and blood flow are not dependent on serum thyroid hormone levels: studies in vivo by color flow doppler sonography Fausto Bogazzi, Luigi Bartalena, Sandra Brogioni, Alessandro Burelli, Luca Manetti, Maria Laura Tanda, Maurizio Gasperi and Enio Martino Dipartimento di Endocrinologia e Metabolismo, Ortopedia e Traumatologia, Medicina del Lavoro, University of Pisa, Pisa, Italy (Correspondence should be addressed to E Martino, Dipartimento di Endocrinologia e Metabolismo, Ortopedia e Traumatologia, Medicina del Lavoro, Universita` di Pisa, Ospedale di Cisanello, Via Paradisa, 2, 56122 Pisa, Italy) Abstract Objective: Thyroid blood flow is greatly enhanced in untreated Graves’ disease, but it is not known whether it is due to thyroid hormone excess or to thyroid hyperstimulation by TSH-receptor antibody. To address this issue in vivo patients with different thyroid disorders were submitted to color flow doppler sonography (CFDS). Subjects and methods: We investigated 24 normal subjects, and 78 patients with untreated hyperthy- roidism (49 with Graves’ hyperthyroidism, 24 with toxic adenoma, and 5 patients with TSH-secreting pituitary adenoma (TSHoma)), 19 patients with thyrotoxicosis (7 with thyrotoxicosis factitia, and 12 with subacute thyroiditis), 37 euthyroid patients with goitrous Hashimoto’s thyroiditis, and 21 untreated hypothyroid patients with Hashimoto’s thyroiditis. Results: Normal subjects had CFDS pattern 0 (absent or minimal intraparenchimal spots) and mean intraparenchimal peak systolic velocity (PSV) of 4.8 6 1.2 cm/s. Patients with spontaneous hyperthy- roidism due to Graves’ disease, TSHoma, and toxic adenoma had significantly increased PSV (P < 0.0001, P = 0.0004, P < 0.0001 respectively vs controls) and CFDS pattern. -

Endocrine Module (PYPP 5260), Thyroid Section, Spring 2002
Endocrine Module (PYPP 5260), Thyroid Section, Spring 2002 THYROID HORMONE TUTORIAL: THYROID PATHOLOGY Jack DeRuiter I. INTRODUCTION Thyroid disorder is a general term representing several different diseases involving thyroid hormones and the thyroid gland. Thyroid disorders are commonly separated into two major categories, hyperthyroidism and hypothyroidism, depending on whether serum thyroid hormone levels (T4 and T3) are increased or decreased, respectively. Thyroid disease generally may be sub-classified based on etiologic factors, physiologic abnormalities, etc., as described in each section below. More than 13 million Americans are affected by thyroid disease, and more than half of these remain undiagnosed. The American Association of Clinical Endocrinologists (AACE) has initiated a campaign to increase public awareness of thyroid disorders and educate Americans about key periods, from birth to advanced age, when people are at increased risk for developing a thyroid disorder (see below). The diagnosis of thyroid disease can be particularly challenging. Patients often present with vague, general clinical manifestations; in particular, the elderly may not associate the signs and symptoms with a disease process and thus may not bring them to the attention of their primary care provider. The prevalence and incidence of thyroid disorders is influenced primarily by sex and age. Thyroid disorders are more common in women than men, and in older adults compared with younger age groups. The prevalence of unsuspected overt hyperthyroidism and hypothyroidism are both estimated to be 0.6% or less in women, based on several epidemiologic studies. Age is also a factor; for overt hyperthyroidism, the prevalence rate is 1.4% for women aged 60 or older and 0.45% for women aged 40 to 60. -

Thyroid Emergencies
REVIEW ARTICLE Thyroid emergencies Dorina Ylli1, Joanna Klubo ‑Gwiezdzinska2, Leonard Wartofsky3,4 1 Endocrinology Division, University of Medicine, Tirana, Albania 2 National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, Maryland, United States 3 Endocrinology Division, Department of Medicine, Georgetown University School of Medicine, Washington DC, United States 4 MedStar Health Research Institute, MedStar Washington Hospital Center, Washington DC, United states KEY WORDS ABSTRACT coma, critical care, Myxedema coma and thyroid storm are among the most common endocrine emergencies presenting to hypothermia, general hospitals. Myxedema coma represents the most extreme, life ‑threatening expression of severe hypothyroidism, hypothyroidism, with patients showing deteriorating mental status, hypothermia, and multiple organ thyrotoxicosis system abnormalities. It typically appears in patients with preexisting hypothyroidism via a common pathway of respiratory decompensation with carbon dioxide narcosis leading to coma. Without early and appropriate therapy, the outcome is often fatal. The diagnosis is based on history and physical find‑ ings at presentation and not on any objective thyroid laboratory test. Clinically based scoring systems have been proposed to aid in the diagnosis. While it is a relatively rare syndrome, the typical patient is an elderly woman (thyroid hypofunction being much more common in women) who may or may not have a history of previously diagnosed or treated thyroid dysfunction. Thyrotoxic storm or thyroid crisis is also a rare condition, established on the basis of a clinical diagnosis. The diagnosis is based on the pres‑ ence of severe hyperthyroidism accompanied by elements of systemic decompensation. Considering that mortality is high without aggressive treatment, therapy must be initiated as early as possible in a critical care setting. -

Uncommon Causes of Thyrotoxicosis*
CONTINUING EDUCATION Uncommon Causes of Thyrotoxicosis* Erik S. Mittra1, Ryan D. Niederkohr1, Cesar Rodriguez1, Tarek El-Maghraby2,3, and I. Ross McDougall1 1Division of Nuclear Medicine and Molecular Imaging Program at Stanford, Department of Radiology, Stanford University Hospital and Clinics, Stanford, California; 2Nuclear Medicine, Cairo University, Cairo, Egypt; and 3Nuclear Medicine, Saad Specialist Hospital, Al Khobar, Saudi Arabia Several of the conditions are self-limiting and do not need Apart from the common causes of thyrotoxicosis, such as prolonged treatment. Graves’ disease and functioning nodular goiters, there are When a patient is thought to be thyrotoxic, a convenient more than 20 less common causes of elevated free thyroid hor- algorithm is to measure free thyroxine (free T ) and mones that produce the symptoms and signs of thyrotoxicosis. 4 thyrotropin (TSH). When the former is higher than normal This review describes these rarer conditions and includes 14 il- lustrative patients. Thyrotropin and free thyroxine should be but the latter is suppressed, thyrotoxicosis is diagnosed. measured and, when the latter is normal, the free triiodothyronine When the former is normal but TSH is low, it is valuable to 123 level should be obtained. Measurement of the uptake of Iis measure free triiodothyronine (free T3); when the latter is recommended for most patients. abnormally high, the diagnosis is T3 toxicosis (2–4). When Key Words: thyrotoxicosis; Graves’ disease; thyroiditis; thyroid both free hormones are normal but TSH is low, the term hormones ‘‘subclinical thyrotoxicosis’’ can be applied (5). Once it has J Nucl Med 2008; 49:265–278 been determined that thyrotoxicosis is present, measure- DOI: 10.2967/jnumed.107.041202 ment of 123I uptake can differentiate among several disor- ders (Table 1). -
15.Thyroid Disorders.Pdf
Thyroid disorders Objectives : 1. Thyroid anatomy and physiology 2. Action of thyroid hormones 3. Thyroid function 4. Thyroid disorders: a. Goiter b. Hyperthyroidism c. Hypothyroidism Done by : Team leader: Salem Al-Ammari Team members: - Mohammed Alswoaiegh - Abdullah Alzaid - Saif almeshari - Majd Albarrak Revised by : Aseel Badukhon Resources : Doctor’s slides + Team 436 Lecturer: Prof. Assim Alfadda & Dr. Aishah Ekhzaimy Same as 436 slides: Yes Step up Important Notes Golden Notes Extra Book Thyroid gland ● Thyroid gland is made up of follicles ● Has 2 lobes and connected by the isthmus ● Weigh 20 g, more volume in men, increase with age and bodyweight and decrease with iodine intake ● Located in front of larynx Thyroid hormone ● Somatic development in adults ● Brain development in infants ● Fetal thyroid functions at 10-12 weeks of gestaion ● Maternal T4 reaches the fetus during development, if mother has hypothyroidism------------ preterm delivery, miscarriage, cognitive impairment of infant ● Main action of thyroid hormones by T3 : 80 % from peripheral conversion and 20 % produced by the thyroid itself. Follicular cells of the thyroid is the main site of hormones synthesis ● Mainly T4 and small amount of T3 ● Iodine is needed to produce thyroid hormones ● Average adult requirement of iodine is 150 mcg a day, 220 mcg for pregnants, 290 mcg for lactating ● Source of iodine: dairy and seafood products Stored in the thyroglobulin in follicular cells of Thyroid hormones synthesis the thyroid gland ● 99.9 % of T4 and T3 are bound to -

Family Practice
THE JOURNAL OF FAMILY PRACTICE Taryn Taylor, MD, CCFP What caused this case of Charles Czarnowski, MD, CCFP asymptomatic hyperthyroidism? Bruyere Family Medicine Center, University of Ottawa Everything pointed to an exogenous cause, but our patient [email protected] denied taking anything. Only later did she mention a diet aid. Practice recommendations stimulating hormone (TSH) test to rule • When taking a medication history, always out hypothyroidism. ask specifi cally about the use of all The test showed a TSH of 0.2 mIU/L nonprescription products—including all (normal range is 0.35-5.0 mIU/L). Her over-the-counter remedies, vitamins, physician ordered retesting a week later “natural” herbal supplements, and dietary and this time, Mary’s TSH was nor- aids (C). mal (1.99 mIU/L). The laboratory report also showed elevated free triiodothyro- • Counsel patients about the need for nine (T3) of 8.1 pmol/L (normal range caution when taking dietary supplements 2.6-5.7 pmol/L) and free thyroxine (T4) and herbal remedies, which lack regulation >70 pmol/L (normal, 10-20 pmol/L); and standardization and may contain negative antithyroid peroxidase and FAST TRACK ingredients not listed on the label (A). antithyroglobulin antibodies; normal Our patient complete blood count, calcium, and Strength of recommendation (SOR) alkaline phosphatase; and low levels hadn’t mentioned A Good-quality patient-oriented evidence of thyroglobulin. The patient had no the European B Inconsistent or limited-quality patient-oriented evidence C Consensus, usual practice, opinion, disease-oriented symptoms and no personal or family dietary evidence, case series history of thyroid disease. -

NACB LMPG Thyroid Disease
Volume 13/2002 The National Academy of Clinical Biochemistry Presents LABORATORY MEDICINE PRACTICE GUIDELINES LABORATORY SUPPORT FOR THE DIAGNOSIS OF THYROID DISEASE ARCHIVED NACB: Laboratory Support for the Diagnosis and Monitoring of Thyroid Disease Laurence M. Demers, Ph.D., F.A.C.B.and Carole A. Spencer Ph.D., F.A.C.B. LABORATORY MEDICINE PRACTICE GUIDELINES Laboratory Support for the Diagnosis and Monitoring of Thyroid Disease Table of Contents Section I. Foreword and Introduction Section 2. Pre-analytic factors Section 3. Thyroid Tests for the Laboratorian and Physician A. Total Thyroxine (TT4) and Total Triiodothyronine (TT3) methods B. Free Thyroxine (FT4) and Free Triiodothyronine (FT3) tests C. Thyrotropin/ Thyroid Stimulating Hormone (TSH) measurement D. Thyroid Autoantibodies: • Thyroid Peroxidase Antibodies (TPOAb) • Thyroglobulin Antibodies (TgAb) • Thyrotrophin Receptor Antibodies (TRAb) E. Thyroglobulin (Tg) Measurement F. Calcitonin (CT) and ret Proto-oncogene G. Urinary Iodide Measurement H. Thyroid Fine Needle Aspiration (FNA) and Cytology I. Screening for Congenital Hypothyroidism Section 4. The Importance of the Laboratory - Physician Interface Appendices and Glossary References Editors: Laurence M. Demers, Ph.D., F.A.C.B. Carole A. Spencer Ph.D., F.A.C.B. Guidelines Committee: The preparation of this revised monograph was achieved with the expert input of the editors, members of the guidelines committee, experts who submitted manuscripts for each section and many expert reviewers, who are listed in Appendix A. The material in this monograph represents the opinions of the editors and does not represent the official position of the National Academy of Clinical Biochemistry or any of the co-sponsoring organizations. The National Academy of Clinical Biochemistry is the official academy of the American Association of Clinical Chemistry. -

Silent Thyroiditis
CLINICAL REVIEW Silent Thyroiditis Roland Sakiyama, MD Los Angeles, California Silent thyroiditis is an increasingly recognized cause of transient thyrotoxicosis. Inflammatory destruction of thyroid follicles results in release of preformed thyroxine and triiodothyronine. Patients present with symptoms of thyrotoxicosis, but unlike subacute thyroiditis, lack thyroid pain or ten derness. The thyrotoxic state spontaneously resolves in 2 to 12 weeks at which time the patient either returns to a euthyroid state or passes through a transient hypothyroid phase. Diagnostic laboratory findings include eleva tions of thyroxine and triiodothyronine and a markedly depressed radioactive iodine uptake. It is imperative for the primary care physician to distinguish silent thyroiditis from chronic causes of hyperthyroidism, eg, Graves' dis ease, since treatment must be palliative rather than definitive. Long-term prognosis is usually excellent. ver the past decade a newly recognized subacute majority of cases of hyperthyroidism. Silent thyroiditis O thyroid disorder has emerged as an important now accounts for upwards of 20 to 30 percent of newly cause of thyrotoxicosis. First described in 1975 by diagnosed cases of thyrotoxicosis.2'6 While growing Gluck et al,1 this disease, like classical subacute physician awareness may account for part of the in thyroiditis, is characterized by inflammation and dis creased incidence, this silent form of subacute thy ruption of normal thyroid architecture resulting in the roiditis appears to be occurring with increased fre release of preformed stores of thyroid hormone. Un quency.2 like subacute thyroiditis, however, patients lack Silent thyroiditis afflicts women more commonly thyroid pain or tenderness. This subacute form of than men in an approximately 2:1 to 3:1 ratio. -

Hypo/Hyperthyroid, Thyroiditis, Thyroid Nodules, Thyroid CA
Schecter Conference Hypo/Hyperthyroid, Thyroiditis, Thyroid nodules, Thyroid CA Ginger Xu PGY-3 General Surgery Oct 27, 2010 Thyroid Embyrology and Anatomy • Develops as a median endodermal downgrowth from the first and second pharyngeal pouches, migrates caudally, then contacts the ultimobranchial bodies developing from the fourth pharyngeal pouches • When it reaches the position it occupies in the adult, with the isthmus situated just below the cricoid cartilage, the thyroid divides into two lobes • The path the gland follows may result in thyroglossal remnants (cysts) or ectopic thyroid tissue (lingual thyroid). • A pyramidal lobe is frequently present. • Agenesis of one thyroid lobe, almost always the left, may occur. • The normal thyroid weighs 15–25 g and is attached to the trachea by loose connective tissue. • highly vascularized organ, blood supply principally from the superior and inferior thyroid arteries. Also possible thyroid ima artery. The recurrent laryngeal nerve runs in the tracheoesophageal groove on the left and has a slightly more oblique course on the right before it enters the larynx just posterior to the cricothyroid muscle at the level of the cricoid cartilage. Physiology - function -> to synthesize, store, and secrete the thyroid hormones (TH): thyroxine (T4) and triiodothyronine (T3) - Iodide is absorbed from the GI tract, actively trapped by the acinar cells of the thyroid, then oxidized and combined with tyrosine in thyroglobulin to form monoiodotyrosine (MIT) and diiodotyrosine (DIT) -MIT and DIT are coupled to form the active hormones T4 and T3, initially stored in the colloid of the gland -Following hydrolysis of the thyroglobulin, T4 and T3 are secreted into the plasma, becoming almost instantaneously bound to plasma proteins - T3 in euthyroid individuals, however, is produced by extrathyroidal conversion of T4 to T3.