Pharmacological Potentials and Toxicity Effects of Excoecaria Agallocha
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Excoecaria Agallocha L
ANNEE : 2015 THESES 2015/ TOU3/ 2020 THESE POUR LE DIPLOME D'ETAT DE DOCTEUR EN PHARMACIE Présentée et soutenue publiquement par ALBINET Lucile Excoecaria agallocha L. Le 16 Mars 2015 Directrice de thèse : VANSTEELANDT Marieke JURY Président : FABRE, Nicolas 1er assesseur : VANSTEELANDT, Marieke 2ème assesseur : AMOUROUX, Noël 1 REMERCIEMENTS Au Président du jury : Mr Fabre, Professeur des Universités à la Faculté des Sciences Pharmaceutiques de Toulouse, je vous remercie de votre disponibilité et d’avoir accepté la présidence de ce jury. Aux membres du jury : Mme Vansteelandt, Maître de Conférences des Universités, et directrice de cette thèse je vous remercie de m’avoir accompagnée dans ce travail et pour votre aide précieuse tout au long de cette thèse. Mr Amouroux, Pharmacien et intervenant à la Faculté de Pharmacie, je vous remercie d’avoir accepté avec spontanéité de faire parti du jury de cette thèse ainsi que pour votre pédagogie tout au long de nos études. A mes parents, qui m’ont toujours encouragée et soutenue. A ma sœur, mon frère, Clau et Jean–Christophe merci pour votre présence et votre soutien. Et comme on dit : « La famille, c’est important la famille ! » A mes amies, Philippine et Sandrine que j’ai eu l’honneur de rencontrer grace à nos études de pharmacie, et dont l’amitié durera encore pour longtemps. Et merci à tous mes amis qui m’accompagnent au quotidien. Enfin, MERCI Rémi pour ton amour et ta grande patience qui, face à cette thèse, ont été mis à rude épreuve mais qui ne t’a pas empêché de vouloir m’épouser ! De tout mon cœur merci. -

Stillingia: a Newly Recorded Genus of Euphorbiaceae from China
Phytotaxa 296 (2): 187–194 ISSN 1179-3155 (print edition) http://www.mapress.com/j/pt/ PHYTOTAXA Copyright © 2017 Magnolia Press Article ISSN 1179-3163 (online edition) https://doi.org/10.11646/phytotaxa.296.2.8 Stillingia: A newly recorded genus of Euphorbiaceae from China SHENGCHUN LI1, 2, BINGHUI CHEN1, XIANGXU HUANG1, XIAOYU CHANG1, TIEYAO TU*1 & DIANXIANG ZHANG1 1 Key Laboratory of Plant Resources Conservation and Sustainable Utilization, South China Botanical Garden, Chinese Academy of Sciences, Guangzhou 510650, China 2University of Chinese Academy of Sciences, Beijing 100049, China * Corresponding author, email: [email protected] Abstract Stillingia (Euphorbiaceae) contains ca. 30 species from Latin America, the southern United States, and various islands in the tropical Pacific and in the Indian Ocean. We report here for the first time the occurrence of a member of the genus in China, Stillingia lineata subsp. pacifica. The distribution of the genus in China is apparently narrow, known only from Pingzhou and Wanzhou Islands of the Wanshan Archipelago in the South China Sea, which is close to the Pearl River estuary. This study updates our knowledge on the geographic distribution of the genus, and provides new palynological data as well. Key words: Island, Hippomaneae, South China Sea, Stillingia lineata Introduction During the last decade, hundreds of new plant species or new species records have been added to the flora of China. Nevertheless, newly described or newly recorded plant genera are not discovered and reported very often, suggesting that botanical expedition and plant survey at the generic level may be advanced in China. As far as we know, only six and eight angiosperm genera respectively have been newly described or newly recorded from China within the last ten years (Qiang et al. -

Dr Pratima S Jadhav* Review Paper Biochemistry
Review Paper Volume-8 | Issue-1 | January-2018 | PRINT ISSN - 2249-555X Biochemistry EXCOECARIA AGALLOCHA: PHYTOCHEMISTRY AND PHARMACOLOGICAL APPLICATIONS Poonam M B Salve Institute of Science, Department of Biochemistry, Mumbai. Dr Pratima S Institute of Science, Department of Biochemistry, Mumbai. *Corresponding Author Jadhav* ABSTRACT Excoecaria agallocha a milky mangrove, blind your eye mangrove, or a river poison tree is widely distributed in Indian coastal regions. This article deals with the overview of Excoecaria, its phytochemical constituents pharmacological applications and its various medicinal uses. The extract of leaves and stems has different phytochemicals as polyphenols, terpenoids, flavonoids, alkaloids and volatile components. The ethanolic and methanolic extract of leaves isolate various compounds. There are antioxidant, antimicrobial, anti-inflammatory, analgesic, antiulcer, anticancer, antireverse transcriptase, antihistamine-release, antifilarial, DNA damage protective, antidiabetic, and antitumor protecting activities carried out on this plant. This review could help the researchers to undertake the further investigations in these directions. KEYWORDS : Mangroves, phytochemicals, pharmacology. Introduction: MS, howed the presence of dodecanediol, L-alanine-4-nitroanilide, Mangroves occur as tall forests through shrub lands in the intertidal benzene methanol, 1,1-diethoxyundecane, hexadecane, Metaraminol, zone along those parts of the coast subject to low wave energy1. 1,2-benzenediol, tetradecane, hexadecane, benzyl alcohol, Mangrove swamps form a type of coastal wetland founad in the tropics benzenemethanol, 4-trifluoroacet benzyl alcohol, L-alanine -4- and subtropics. They act as a source of energy in coastal food chain and nitroanilide, alanine, 2,6-Octadiene-4, undecane, Pentanoic acid, also protects against various natural calamities such as cyclone and hydroxybenzenepropanoic acid, diethyl methylphosphonate, acridine, tsunami2. -

Excoecaria Agallocha L. Antimicrobial Properties Against Important Pathogenic Microorganisms
International Journal of ChemTech Research CODEN( USA): IJCRGG ISSN : 0974-4290 Vol.1, No.4, pp 865-867, Oct-Dec 2009 EXCOECARIA AGALLOCHA L. ANTIMICROBIAL PROPERTIES AGAINST IMPORTANT PATHOGENIC MICROORGANISMS Varahalarao Vadlapudi1, Varaprasad Bobbarala1*, Somasekhar Penumajji2, K. Chandrasekhar Naidu1 1Department of Botany, Andhra University, Visakhapatnam-3, A.P.,India. 2Vivimed labs Limited, 2nd, 4th Floor, Veeranag towers, Habsiguda, Hyderabad, A.P.,India. * Corresponding Author: [email protected] ABSTRACT: Excoecaria agallocha L. leaves were extracted by various extracting procedures, using different solvents for testing the antimicrobial activities against important microorganisms using agar well diffusion method. Chloroform and methanolic extracts were found to be effective against these organisms, whereas hexane extracts were inactive. The purpose of this study was to find preliminary data for the development of alternative treatments to chemical microbicides for the control of plant diseases from natural plant extracts. Keywords: Excoecaria agallocha, Agar well diffusion method; Antimicrobial activity. INTRODUCTION phytochemical and bioactivity studies on mangrove Medical plants have been used for years in daily plants from Kakinada and Godavari, we now report life to treat disease all over the world. It is well known assessment of in vitro antimicrobial activity including that some plants containing active compounds are able to pathogenic bacterial and fungal strains. inhibit the microbial growth. The potential of antimicrobial properties of plants are related to their MATERIALS AND METHODS ability to synthesize compounds by the secondary E. agallocha L. commonly known as milky metabolism. Secondary metabolites proved to be the most mangrove and its vernacular name is Tilla and this important group of compounds that showed wide range species of mangrove tree classified in the plant family of antibacterial and antifungal activity. -

Original Article ANTINOCICEPTIVE and GASTROPROTECTIVE
Turk J. Pharm. Sci. 5 (3) 143-154, 2008 Original Article ANTINOCICEPTIVE AND GASTROPROTECTIVE EFFECT OF THE CRUDE ETHANOLIC EXTRACTS OF EXCOECARIA AGALLOCHA LINN. Nusrat SUBHAN1, Ashraful ALAM2*, Firoj AHMED3, Israt Zahan SHAHİD3 1 Northern University, Department of Pharmacy, Dhaka, BANGLADESH 2 Stamford University, Department of Pharmacy, Dhaka, BANGLADESH 3 Khulna University, Pharmacy Discipline, Phytochemical and Pharmacognosy Laboratory, Khulna, BANGLADESH Abstract The effect of alcoholic extracts of bark from Excoecaria. agallocha Linn. (Family: Euphorbiaceae) was evaluated in experimental models of pain and ulceration. Crude extracts of Excoecaria agallocha (300 mg/kg dose) showed maximum time needed for the response against thermal stimuli (6.72±0.43 seconds) which is comparable to diclofenac sodium (8.20±0.21seconds) in the hot plate test. Hot tail immersion test also showed similar results as in hot plate test. The bark extracts at 500 and 250 mg/kg showed significant reduction in acetic acid induced writhings in mice with a maximum effect of 53.87% reduction at 500 mg/kg dose. The effect produced by the alcoholic extract at the highest dose was comparable to that of diclofenac sodium at 100 mg/kg (70.56%). It has also been seen anti-ulcerogenic activity compared to acetylsalicylic acid, which may be due to the protective effect of the extract. The result suggest that the analgesic effect of the extract as claimed in folklore medicine, which may be mediated via both peripheral and central mechanism having gastro-protective effect. Key Words: Excoecaria agallocha, Euphorbiaceae, hot plate test, gastric protection. Excoecaria Agallocha Linn. in Etanoldeki Ham Ekstresinin Antinosisieptif Gastroprotektif Etkisi Excoecaria agallocha Linn. -

Southern Gulf, Queensland
Biodiversity Summary for NRM Regions Species List What is the summary for and where does it come from? This list has been produced by the Department of Sustainability, Environment, Water, Population and Communities (SEWPC) for the Natural Resource Management Spatial Information System. The list was produced using the AustralianAustralian Natural Natural Heritage Heritage Assessment Assessment Tool Tool (ANHAT), which analyses data from a range of plant and animal surveys and collections from across Australia to automatically generate a report for each NRM region. Data sources (Appendix 2) include national and state herbaria, museums, state governments, CSIRO, Birds Australia and a range of surveys conducted by or for DEWHA. For each family of plant and animal covered by ANHAT (Appendix 1), this document gives the number of species in the country and how many of them are found in the region. It also identifies species listed as Vulnerable, Critically Endangered, Endangered or Conservation Dependent under the EPBC Act. A biodiversity summary for this region is also available. For more information please see: www.environment.gov.au/heritage/anhat/index.html Limitations • ANHAT currently contains information on the distribution of over 30,000 Australian taxa. This includes all mammals, birds, reptiles, frogs and fish, 137 families of vascular plants (over 15,000 species) and a range of invertebrate groups. Groups notnot yet yet covered covered in inANHAT ANHAT are notnot included included in in the the list. list. • The data used come from authoritative sources, but they are not perfect. All species names have been confirmed as valid species names, but it is not possible to confirm all species locations. -
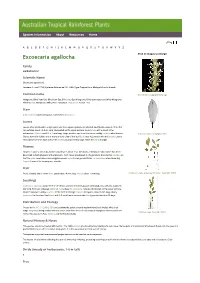
Excoecaria Agallocha Click on Images to Enlarge
Species information Abo ut Reso urces Hom e A B C D E F G H I J K L M N O P Q R S T U V W X Y Z Excoecaria agallocha Click on images to enlarge Family Euphorbiaceae Scientific Name Excoecaria agallocha L. Linnaeus, C. von (1759) Systema Naturae ed. 10 : 1288. Type: Tropical Asia, Malaya & Pacific Islands. Common name Male flowers. Copyright Barry Jago Mangrove, Blind Your Eye; Blind Your Eye; Blind Your Eye Mangrove; Blind-your-eyes-tree; Milky Mangrove; Blinding Tree; Mangrove, Milky; River Poisonous Tree; Scrub Poison Tree Stem Bark exudate rapid and copious. Sometimes deciduous. Leaves Leaves often produced in a tight spiral and thus appear opposite or whorled. Leaf blades about 6-10.5 x 3-5 cm, petioles about 1.5-2 cm long, channelled on the upper surface. Apex retuse with a gland in the indentation. Stipules small, 1.5-2 mm long. Twigs, petioles and leaves produce a milky exudate when broken. Scale bar 10mm. Copyright CSIRO Glands normally visible, one or more on each side of the leaf blade near its junction with the petiole. Lateral veins about 12-18 on each side of the midrib usually forming loops inside the blade margin. Flowers Flowers in spikes, about 40-70 mm long, flowers about 1.5-2 mm diam., emitting an odour which has been described as both pleasant and unpleasant. Each flower enveloped in a bi-glandular bract before anthesis so that the spike resembles a narrow gymnosperm cone. Pollen yellow. Plants +/- deciduous when flowering. -

Fl. China 11: 280–282. 2008. 65. EXCOECARIA Linnaeus, Syst. Nat
Fl. China 11: 280–282. 2008. 65. EXCOECARIA Linnaeus, Syst. Nat., ed. 10, 2: 1288. 1759. 海漆属 hai qi shu Li Bingtao (李秉滔 Li Ping-tao); Hans-Joachim Esser Commia Loureiro. Trees or shrubs, with milky juice, glabrous. Leaves alternate or opposite, petiolate; stipules small, caducous; leaf blade entire or serrulate, penninerved. Flowers unisexual (plants monoecious or dioecious), apetalous, without disk, in axillary or terminal racemelike thyrses. Male flowers (sub)sessile; sepals 3, small, imbricate, free; stamens 3; filaments free; anthers longitudinally dehiscent, without pistillode. Female flowers sessile to pedicellate; calyx 3-lobed or 3-partite; ovary 3-celled, smooth; ovules 1 per locule; stigmas extended or recurved, free to slightly connate at base, undivided, eglandular. Capsules dehiscent into 2-valved cocci; columella persistent, winged. Seeds globose, estrophiolate; episperm crustaceous; endosperm fleshy; cotyledon broad and flattened. About 35 species: Africa, Asia, Australia, Pacific islands; five species (two endemic) in China. 1a. Leaves opposite above, alternate on lower parts. 2a. Leaf blade purple or dark red abaxially........................................................................................................ 1. E. cochinchinensis 2b. Leaf blade green or greenish abaxially when old. 3a. Leaf blade ca. 3 × as long as wide, apex acuminate, not falcate, petiole 3–13 mm ............................ 1. E. cochinchinensis 3b. Leaf blade more than 5 × as long as wide, apex acuminate-falcate, petiole 3–5 mm .................................... 2. E. venenata 1b. Leaves alternate on all parts. 4a. Leaf blade serrulate; male bracts 2- or 3-flowered ............................................................................................... 5. E. acerifolia 4b. Leaf blade entire or nearly so; male bracts 1-flowered. 5a. Petioles 2-glandular at apex; plants dioecious ................................................................................................ 3. E. agallocha 5b. -

Fl. China 11: 284–285. 2008. 69. TRIADICA Loureiro, Fl. Cochinch. 2
Fl. China 11: 284–285. 2008. 69. TRIADICA Loureiro, Fl. Cochinch. 2: 598, 610. 1790. 乌桕属 wu jiu shu Li Bingtao (李秉滔 Li Ping-tao); Hans-Joachim Esser Sapium sect. Triadica (Loureiro) Müller Argoviensis. Trees or shrubs, monoecious or sometimes dioecious; indumentum absent; latex white. Leaves alternate or nearly opposite; petioles with 1 or 2 apical glands; leaf blade margin entire or serrate; venation pinnate, lowermost pair of veins originating at very leaf base, forming basal margin. Inflorescences terminal or axillary, spikelike or racemelike thyrses, sometimes branched; bracts with 2 large abaxial glands at base. Male flowers small, yellow, fascicled in axils of bracts; calyx membranous, cup-shaped, shallowly 2- or 3-lobed or -dentate; petals absent; disk absent; stamens 2–3; filaments free; anthers 2-celled, longitudinally dehiscent; pistillode absent. Female flowers larger than male, 1 per bract; calyx cup-shaped, 3-partite, or cylindric and 3-dentate, rarely 2- or 3-sepaled; petals absent; disk absent; ovary 2- or 3-celled; ovules 1 per cell; styles usually 3, free or connate at base; stigma revolute, entire. Capsules globose, pyriform or 3-valved, rarely baccate, usually 3-celled, loculicidal, sometimes irregularly dehiscent. Seeds subglobose, usually covered by waxy aril; exocarp hard; endosperm fleshy; cotyledon broad and flattened. Three species: E and S Asia; three species in China. 1a. Petiole with a single gland above; leaf blade subrotund, base cordate to rounded, apex rounded or rarely acute or incised .................................................................................................................................................................... 3. T. rotundifolia 1b. Petiole with a pair of glands above; leaf blade ovate to elliptic, base cuneate to obtuse (very rarely rounded), apex acute to acuminate. -

Downloaded from Brill.Com10/09/2021 12:24:23AM Via Free Access 2 IAWA Journal, Vol
IAWA Journal, Vol. 26 (1), 2005: 1-68 WOOD ANATOMY OF THE SUBFAMILY EUPHORBIOIDEAE A comparison with subfamilies Crotonoideae and Acalyphoideae and the implications for the circumscription of the Euphorbiaceae Alberta M. W. Mennega Nationaal Herbarium Nederland, Utrecht University branch, Heidelberglaan 2, 3584 es Utrecht, The Netherlands SUMMARY The wood anatomy was studied of 82 species from 34 out of 54 genera in the subfamily Euphorbioideae, covering all five tribes recognized in this subfamily. In general the woods show a great deal of similarity. They are charac terized by a relative paucity of vessels, often arranged in short to long, dumbbell-shaped or twin, radial multiples, and by medium-sized to large intervessel pits; fibres often have gelatinous walls; parenchyma apotracheal in short, wavy, narrow bands and diffuse-in-aggregates; mostly uni- or only locally biseriate rays, strongly heterocellular (except Hippomane, Hura and Pachystroma). Cell contents, either silica or crystals, or both together, are nearly always present and often useful in distinguishing between genera. Radiallaticifers were noticed in most genera, though they are scarce and difficult to trace. The laticifers are generally not surrounded by special cells, except in some genera of the subtribe Euphorbiinae where radiallaticifers are comparatively frequent and conspicuous. Three ofthe five tribes show a great deal of conformity in their anatomy. Stomatocalyceae, however, stand apart from the rest by the combination of the scarcity of vessels, and mostly biseriate, vertically fused and very tall rays. Within Euphorbieae the subtribe Euphorbiinae shows a greater vari ation than average, notably in vessel pitting, the frequent presence of two celled parenchyma strands, and in size and frequency of the laticifers. -

Insight on Excoecaria Agallocha
s Chemis ct try u d & o r R P e s l e Kaliamurthi and Selvaraj, Nat Prod Chem Res 2016, 4:2 a r a r u t c h a N Natural Products Chemistry & Research DOI: 10.4172/2329-6836.1000203 ISSN: 2329-6836 Research Article Open Access Insight on Excoecaria agallocha: An Overview Satyavani Kaliamurthi* and Gurudeeban Selvaraj Centre of Advanced Study in Marine Biology, Annamalai University, Tamil Nadu, India Abstract Excoecaria agallocha is a milky mangrove widely distributed in Indian coastal regions. This review article explains chemical composition, pharmaceutical and environmental applications of E. agallocha. There are 20 different polyphenols, 15 terpenoids and more than 50 volatile derivatives were identified from leaves, stem, latex and root extract. Enormous number of compounds isolated from ethanolic extact of leaves. In conclusion, E. agallocha has huge amount of polyphenols and terpenoids, which was reported to have endocrine, epidemic and endemic disease control as anti-microbial, anti-cancer and anti-diabetic agent. Keywords: Mangroves; Thillai; Terpenoids; Rutin; Antidiabetic insulin and glucagon. The islets of Langerhans secrete insulin and glucagon directly into the blood [7]. When the blood glucose level falls, Background glucagon secreted and increases blood glucose concentration partly by A mangrove is a tree, shrub, palm or ground fern, generally breaking down stored glycogen in the liver by a glycogenolysis pathway. exceeding one half meter in height, that normally grows above mean Also, Gluconeogenesis is the production of glucose in the liver from sea level in the intertidal zone of marine coastal environments and non-carbohydrate precursors such as glycogenic amino acids [8]. -

Mangrove Guidebook for Southeast Asia
RAP PUBLICATION 2006/07 MANGROVE GUIDEBOOK FOR SOUTHEAST ASIA The designations and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations concerning the legal status of any country, territory, city or area or of its frontiers or boundaries. The opinions expressed in this publication are those of the authors alone and do not imply any opinion whatsoever on the part of FAO. Authored by: Wim Giesen, Stephan Wulffraat, Max Zieren and Liesbeth Scholten ISBN: 974-7946-85-8 FAO and Wetlands International, 2006 Printed by: Dharmasarn Co., Ltd. First print: July 2007 For copies write to: Forest Resources Officer FAO Regional Office for Asia and the Pacific Maliwan Mansion Phra Atit Road, Bangkok 10200 Thailand E-mail: [email protected] ii FOREWORDS Large extents of the coastlines of Southeast Asian countries were once covered by thick mangrove forests. In the past few decades, however, these mangrove forests have been largely degraded and destroyed during the process of development. The negative environmental and socio-economic impacts on mangrove ecosystems have led many government and non- government agencies, together with civil societies, to launch mangrove conservation and rehabilitation programmes, especially during the 1990s. In the course of such activities, programme staff have faced continual difficulties in identifying plant species growing in the field. Despite a wide availability of mangrove guidebooks in Southeast Asia, none of these sufficiently cover species that, though often associated with mangroves, are not confined to this habitat.