Journal Arnold Arboretum
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CHAPTER 2 REVIEW of the LITERATURE 2.1 Taxa And
CHAPTER 2 REVIEW OF THE LITERATURE 2.1 Taxa and Classification of Acalypha indica Linn., Bridelia retusa (L.) A. Juss. and Cleidion javanicum BL. 2.11 Taxa and Classification of Acalypha indica Linn. Kingdom : Plantae Division : Magnoliophyta Class : Magnoliopsida Order : Euphorbiales Family : Euphorbiaceae Subfamily : Acalyphoideae Genus : Acalypha Species : Acalypha indica Linn. (Saha and Ahmed, 2011) Plant Synonyms: Acalypha ciliata Wall., A. canescens Wall., A. spicata Forsk. (35) Common names: Brennkraut (German), alcalifa (Brazil) and Ricinela (Spanish) (36). 9 2.12 Taxa and Classification of Bridelia retusa (L.) A. Juss. Kingdom : Plantae Division : Magnoliophyta Class : Magnoliopsida Order : Malpighiales Family : Euphorbiaceae Genus : Bridelia Species : Bridelia retusa (L.) A. Juss. Plant Synonyms: Bridelia airy-shawii Li. Common names: Ekdania (37,38). 2.13 Taxa and Classification of Cleidion javanicum BL. Kingdom : Plantae Subkingdom : Tracheobionta Superdivision : Spermatophyta Division : Magnoliophyta Class : Magnoliopsida Subclass : Magnoliopsida Order : Malpighiales Family : Euphorbiaceae Genus : Cleidion Species : Cleidion javanicum BL. Plant Synonyms: Acalypha spiciflora Burm. f. , Lasiostylis salicifolia Presl. Cleidion spiciflorum (Burm.f.) Merr. Common names: Malayalam and Yellari (39). 10 2.2 Review of chemical composition and bioactivities of Acalypha indica Linn., Bridelia retusa (L.) A. Juss. and Cleidion javanicum BL. 2.2.1 Review of chemical composition and bioactivities of Acalypha indica Linn. Acalypha indica -

Approved Conservation Advice for Actephila Foetida
This Conservation Advice was approved by the Minister / Delegate of the Minister on: 16/12/2008 Approved Conservation Advice (s266B of the Environment Protection and Biodiversity Conservation Act 1999) Approved Conservation Advice for Actephila foetida This Conservation Advice has been developed based on the best available information at the time this Conservation Advice was approved; this includes existing plans, records or management prescriptions for this species. Description Actephila foetida, Family Euphorbiaceae, is a subshrub up to 1 m tall. The young branchlets are densely covered with soft, short hairs. The leaf stalks are 1.8–7.8 cm long and dark olive- green when dry. The thin leaves are broadly elliptic to obovate, measuring 4.5–53 cm long by 3–21.3 cm wide and are alternately arranged along the branchlets. The upper leaf surface is dark olive-green and more or less hairless; the lower surface is pale olive-green, with a dense covering of spreading hairs on the lateral veins. The flowers are unisexual. Male and female flowers are mixed together in clusters borne in leaf axils. The flower clusters measure 7– 13 mm in diameter and the flowers are approximately 4–8 mm in diameter. Both male and female flowers have 5 sepals and a conspicuous fleshy disk. The fruits are depressed-globose in shape, 15–19 mm in diameter and split releasing up to 3 seeds. This species is distinguished by the (usually) large leaves and the hispid indumentum on the lower surface of the leaf and the flowers that lack petals (Forster, 2005). Conservation Status Actephila foetida is listed as vulnerable. -
![Entry for ACALYPHA Acrogyna Pax [Family EUPHORBIACEAE]](https://docslib.b-cdn.net/cover/8673/entry-for-acalypha-acrogyna-pax-family-euphorbiaceae-78673.webp)
Entry for ACALYPHA Acrogyna Pax [Family EUPHORBIACEAE]
Entry for ACALYPHA acrogyna Pax [family EUPHORBIACEAE] http://plants.jstor.org/flora/flota011327 http://www.jstor.org Your use of the JSTOR archive indicates your acceptance of JSTOR's Terms and Conditions of Use, available at http://www.jstor.org/page/info/about/policies/terms.jsp. JSTOR's Terms and Conditions of Use provides, in part, that unless you have obtained prior permission, you may not download an entire issue of a journal or multiple copies of articles, and you may use content in the JSTOR archive only for your personal, non-commercial use. Please contact the contributing partner regarding any further use of this work. Partner contact information may be obtained at http://plants.jstor.org/page/about/plants/PlantsProject.jsp. Each copy of any part of a JSTOR transmission must contain the same copyright notice that appears on the screen or printed page of such transmission. JSTOR is a not-for-profit service that helps scholars, researchers, and students discover, use, and build upon a wide range of content in a trusted digital archive. We use information technology and tools to increase productivity and facilitate new forms of scholarship. For more information about JSTOR, please contact [email protected]. Page 1 of 2 Entry for ACALYPHA acrogyna Pax [family EUPHORBIACEAE] Herbarium Royal Botanic Gardens, Kew (K) Collection Flora of Tropical Africa Resource Type Reference Sources Entry from Flora of Tropical Africa, Vol 6 Part 1, page 441 (1913) Author: (By J. G. Baker, with additions by C. H. Wright.) Names ACALYPHA acrogyna Pax [family EUPHORBIACEAE], in Engl. Jahrb. -
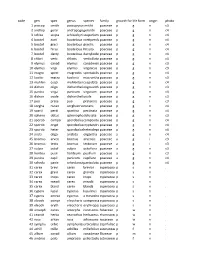
Species List (PDF)
code gen spec genus species family growth formlife form origin photo 1 pascop smith pascopyrumsmithii poaceae p g n c3 2 androp gerar andropogongerardii poaceae p g n c4 3 schiza scopa schizachyriumscoparium poaceae p g n c4 4 boutel curti bouteloua curtipendulapoaceae p g n c4 5 boutel graci bouteloua gracilis poaceae p g n c4 6 boutel hirsu bouteloua hirsuta poaceae p g n c4 7 boutel dacty bouteloua dactyloidespoaceae p g n c4 8 chlori verti chloris verticillata poaceae p g n c4 9 elymus canad elymus canadensispoaceae p g n c3 10 elymus virgi elymus virginicus poaceae p g n c3 11 eragro spect eragrostis spectabilis poaceae p g n c4 12 koeler macra koeleria macrantha poaceae p g n c3 13 muhlen cuspi muhlenbergiacuspidata poaceae p g n c4 14 dichan oligo dichantheliumoligosanthespoaceae p g n c3 15 panicu virga panicum virgatum poaceae p g n c4 16 dichan ovale dichantheliumovale poaceae p g n c3 17 poa prate poa pratensis poaceae p g i c3 18 sorgha nutan sorghastrumnutans poaceae p g n c4 19 sparti pecti spartina pectinata poaceae p g n c4 20 spheno obtus sphenopholisobtusata poaceae p g n c3 21 sporob compo sporoboluscomposituspoaceae p g n c4 22 sporob crypt sporoboluscryptandruspoaceae p g n c4 23 sporob heter sporobolusheterolepispoaceae p g n c4 24 aristi oliga aristida oligantha poaceae a g n c4 25 bromus arven bromus arvensis poaceae a g i c3 26 bromus tecto bromus tectorum poaceae a g i c3 27 vulpia octof vulpia octoflora poaceae a g n c3 28 hordeu pusil hordeum pusillum poaceae a g n c3 29 panicu capil panicum capillare poaceae a g n c4 30 schedo panic schedonnarduspaniculatuspoaceae p g n c4 31 carex brevi carex brevior cyperaceaep s n . -

Richard Chinn Environmental Training, Inc. Info
Scientific Name Common Name Region 6 Habit Scientific Name Common Name Region 6 Habit Abies balsamea FIR,BALSAM FACW NT Amaranthus californicus AMARANTH,CALIFORNIA NI ANF Abutilon theophrasti VELVET-LEAF NI AIF Amaranthus crassipes AMARANTH,TROPICAL FAC+ AIF Acacia greggii ACACIA,CATCLAW UPL NST Amaranthus greggii AMARANTH,GREGGIS FAC ANF Acacia smallii HUISACHE FACU NTS Amaranthus obcordatus AMARANTH,TRANS PECOS NI ANF Acalypha rhomboidea COPPER-LEAF,COMMON UPL* ANF Amaranthus palmeri AMARANTH,PALMER'S FACU- ANF Acalypha virginica MERCURY,THREE-SEEDED UPL* ANF Amaranthus retroflexus AMARANTH,RED-ROOT FACU- ANF Acer negundo BOX-ELDER FACW- NT Amaranthus rudis AMARANTH,TALL FAC ANF Acer rubrum MAPLE,DRUMMOND RED FACW NT Amaranthus spinosus AMARANTH,SPINY FACU- ANF Acer rubrum MAPLE,TRIDENT RED NI NT Amaranthus tuberculatus AMARANTH,ROUGH-FRUIT NI ANF Acer rubrum MAPLE,RED FAC NT Ambrosia artemisiifolia RAGWEED,ANNUAL FACU- ANF Acer saccharinum MAPLE,SILVER FAC NT Ambrosia grayi BURSAGE,WOOLLY-LEAF FACW PNF Acer saccharum MAPLE,SUGAR UPL NT Ambrosia psilostachya RAGWEED,NAKED-SPIKE FAC- PNF Achillea millefolium YARROW,COMMON FACU PNF Ambrosia trifida RAGWEED,GREAT FAC ANF Acorus calamus SWEETFLAG OBL PIEF Amelanchier alnifolia SERVICE-BERRY,SASKATOON FAC- NS Adiantum capillus-veneris FERN,SOUTHERN MAIDEN-HAIR FACW+ PNF3 Amelanchier arborea SERVICE-BERRY,DOWNY FACU NT Adiantum pedatum FERN,NORTHERN MAIDEN-HAIR FAC PNF3 Amianthium muscaetoxicum FLYPOISON FAC PNF Adiantum tricholepis FERN,HAIRY MAIDEN-HAIR FAC PNF3 Ammannia auriculata AMMANNIA,RED-STEM -

Survey of Euphorbiaceae Family in Kopergaon Tehsil Of
International Journal of Humanities and Social Sciences (IJHSS) ISSN (P): 2319–393X; ISSN (E): 2319–3948 Vol. 9, Issue 3, Apr–May 2020; 47–58 © IASET SURVEY OF EUPHORBIACEAE FAMILY IN KOPERGAONTEHSIL OF MAHARASHTRA Rahul Chine 1 & MukulBarwant 2 1Research Scholar, Department of Botany, Shri Sadguru Gangagir Maharaj Science College, Maharashtra, India 2Research Scholar, Department of Botany, Sanjivani Arts Commerce and Science College, Maharashtra, India ABSTRACT The survey of Family Euphorbiaceae from Kopargoantehshil is done. In this we first collection of different member of Family Euphorbiaceae from different region of Kopargoantehasil. An extensive and intensive survey at plants was carried out from village Pathare, Derde, Pohegoan, Kopergaon, Padhegaon, Apegoan during the were collected in flowering and fruiting period throughout the year done. During survey we determine 16 member of Euphorbiceae from Kopargoantehshil Then we decide characterization on the basis of habit, flowering character, leaf and fruit character with help of that character and using different literature we identified each and every member of Euphorbiaceae Species were identified with relevant information and documented in this paper with regard to their Botanical Name, family, Habitat, flowering Fruiting session and their medicinal value of some member of Euphorbiaceae that used in medicine respiratory disorder such as cough, asthama, bronchitis etc and some are toxic in nature due to their toxic latex that is showing itching reaction. KEYWORDS : Family Euphorbiaceae, Respiratory Ailment, Identification, Chracterization and Documentation Article History Received: 09 Apr 2020 | Revised: 10 Apr 2020 | Accepted: 18 Apr 2020 INTRODUCTION The Euphorbiaceae, the spurge family, is one of the complex large family of flowering plants of angiosperm with 334 genera and 8000 species in the worlds (Wurdack 2004). -

Aleurites Fordii Hemsl.) (Euphorbiaceae): New to the Arkansas Flora Brett Es Rviss Henderson State University, [email protected]
Journal of the Arkansas Academy of Science Volume 61 Article 24 2007 Tungoil Tree (Aleurites fordii Hemsl.) (Euphorbiaceae): New to the Arkansas Flora Brett eS rviss Henderson State University, [email protected] Nicole Freeman Henderson State University Joslyn Hernandez Henderson State University Allen Leible Henderson State University Chris Talley Henderson State University Follow this and additional works at: http://scholarworks.uark.edu/jaas Part of the Plant Biology Commons Recommended Citation Serviss, Brett; Freeman, Nicole; Hernandez, Joslyn; Leible, Allen; and Talley, Chris (2007) "Tungoil Tree (Aleurites fordii Hemsl.) (Euphorbiaceae): New to the Arkansas Flora," Journal of the Arkansas Academy of Science: Vol. 61 , Article 24. Available at: http://scholarworks.uark.edu/jaas/vol61/iss1/24 This article is available for use under the Creative Commons license: Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0). Users are able to read, download, copy, print, distribute, search, link to the full texts of these articles, or use them for any other lawful purpose, without asking prior permission from the publisher or the author. This General Note is brought to you for free and open access by ScholarWorks@UARK. It has been accepted for inclusion in Journal of the Arkansas Academy of Science by an authorized editor of ScholarWorks@UARK. For more information, please contact [email protected]. - Journal of the Arkansas Academy of Science, Vol. 61 [2007], Art. 24 Tungoil Tree (Alellritesfordii Hemsl.) (Euphorbiaceae) New to the Arkansas Flora !Henderson State University, Biology Department, P.O Box H-7570, Arkadelphia, AR 71999-0001 ICorrespondence: [email protected] The problems associated with the introduction, subsequent and become invasive in Arkansas and elsewhere in the United establishment, and naturalization ofnon-native plant species in States following intentional introduction. -

Ultramafic Geocology of South and Southeast Asia
Galey et al. Bot Stud (2017) 58:18 DOI 10.1186/s40529-017-0167-9 REVIEW Open Access Ultramafc geoecology of South and Southeast Asia M. L. Galey1, A. van der Ent2,3, M. C. M. Iqbal4 and N. Rajakaruna5,6* Abstract Globally, ultramafc outcrops are renowned for hosting foras with high levels of endemism, including plants with specialised adaptations such as nickel or manganese hyperaccumulation. Soils derived from ultramafc regoliths are generally nutrient-defcient, have major cation imbalances, and have concomitant high concentrations of potentially phytotoxic trace elements, especially nickel. The South and Southeast Asian region has the largest surface occur- rences of ultramafc regoliths in the world, but the geoecology of these outcrops is still poorly studied despite severe conservation threats. Due to the paucity of systematic plant collections in many areas and the lack of georeferenced herbarium records and databased information, it is not possible to determine the distribution of species, levels of end- emism, and the species most threatened. However, site-specifc studies provide insights to the ultramafc geoecology of several locations in South and Southeast Asia. The geoecology of tropical ultramafc regions difers substantially from those in temperate regions in that the vegetation at lower elevations is generally tall forest with relatively low levels of endemism. On ultramafc mountaintops, where the combined forces of edaphic and climatic factors inter- sect, obligate ultramafc species and hyperendemics often occur. Forest clearing, agricultural development, mining, and climate change-related stressors have contributed to rapid and unprecedented loss of ultramafc-associated habitats in the region. The geoecology of the large ultramafc outcrops of Indonesia’s Sulawesi, Obi and Halmahera, and many other smaller outcrops in South and Southeast Asia, remains largely unexplored, and should be prioritised for study and conservation. -

Ethnobotanical Observations of Euphorbiaceae Species from Vidarbha Region, Maharashtra, India
Ethnobotanical Leaflets 14: 674-80, 2010. Ethnobotanical Observations of Euphorbiaceae Species from Vidarbha region, Maharashtra, India G. Phani Kumar* and Alka Chaturvedi# Defence Institute of High Altitude Research (DRDO), Leh-Ladakh, India #PGTD Botany, RTM Nagpur University, Nagpur, India *corresponding author: [email protected] Issued: 01 June, 2010 Abstract An attempt has been made to explore traditional medicinal knowledge of plant materials belonging to various genera of the Euphorbiaceae, readily available in Vidharbha region of Maharasthtra state. Ethnobotanical information were gathered through several visits, group discussions and cross checked with local medicine men. The study identified 7 species to cure skin diseases (such as itches, scabies); 5 species for antiseptic (including antibacterial); 4 species for diarrhoea; 3 species for dysentery, urinary infections, snake-bite and inflammations; 2 species for bone fracture/ dislocation, hair related problems, warts, fish poisons, night blindness, wounds/cuts/ burns, rheumatism, diabetes, jaundice, vomiting and insecticide; 1 species as laxative , viral fever and arthritis. The results are encouraging but thorough scientific scrutiny is absolutely necessary before being put into practice. Key words: Ethnopharmacology; Vidarbha region; Euphorbiaceae; ethnobotanical information. Introduction The medicinal properties of a plant are due to the presence of certain chemical constituents. These chemical constituents, responsible for the specific physiological action, in the plant, have in many cases been isolated, purified and identified as definite chemical compounds. Quite a large number of plants are known to be of medicinal use remain uninvestigated and this is particularly the case with the Indian flora. The use of plants in curing and healing is as old as man himself (Hedberg, 1987). -

Revision of the Genus Cleidion (Euphorbiaceae) in Malesia
BLUMEA 50: 197–219 Published on 22 April 2005 http://dx.doi.org/10.3767/000651905X623373 REVISION OF THE GENUS CLEIDION (EUPHORBIACEAE) IN MALESIA KRISTO K.M. KULJU & PETER C. VAN WELZEN Nationaal Herbarium Nederland, Universiteit Leiden branch, P.O. Box 9514, 2300 RA Leiden, The Netherlands; e-mail: [email protected], [email protected] SUMMARY A revision of the Malesian species in the genus Cleidion is presented. Cleidion javanicum is shown to be the correct name for the widespread type species (instead of the name C. spiciflorum). A new species, C. luziae, resembling C. javanicum, is described from the Moluccas, New Guinea and the Solomon Islands. In addition, C. salomonis is synonymised with C. papuanum and C. lanceolatum is treated as a variety of C. ramosii. In total 7 Malesian Cleidion species are recognized. Cleidion megistophyllum from the Philippines cannot reliably be confirmed to belong to the genus due to lack of information and specimens and is treated as a doubtful species. Key words: Cleidion, Acalypheae, Cleidiinae, revision, taxonomy, Malesia. INTRODUCTION Cleidion is a pantropical genus belonging to the large angiosperm family Euphorbiaceae s.s. It was described by Blume (1825), who included a single species C. javanicum1. The first revision was made by Müller Argoviensis (1865, 1866). His work was fol- lowed by the comprehensive treatment of Pax & Hoffmann (1914), which included 17 species. Pax & Hoffmann excluded the section Discocleidion Müll.Arg. which differs from Cleidion by the presence of a staminate and pistillate disc (in Cleidion a disc is absent), stipellate and palmatinerved leaves (in Cleidion the leaves are non-stipellate and pinnatinerved), and differences in anther type. -

Valorisation of Reutealis Trisperma Seed from Papua for the Production of Non-Edible Oil and Protein-Rich Biomass
International Proceedings of Chemical, Biological and Environmental Engineering, V0l. 93 (2016) DOI: 10.7763/IPCBEE. 2016. V93. 3 Valorisation of Reutealis Trisperma Seed from Papua for the Production of Non-Edible Oil and Protein-Rich Biomass Robert Manurung 1, Muhammad Yusuf Abduh 1, Mochammad Hirza Nadia 1, Kardina Sari Wardhani 1, and Khalilan Lambangsari 1 1 School of Life Sciences and Technology, Institut Teknologi Bandung, Indonesia Abstract. The valorisation of Reutealis trisperma seed for the production of non-edible oil and protein was investigated. Reutealis trisperma fruits contain approximately 60-61 wt%, d.b. mesocarp, 26-28 wt%, d.b. endosperm and 13 wt%, d.b. endocarp. The endosperm of ripe Reutealis trisperma fruit contains about 54-59 wt%, d.b. non-edible oil whereas the mesocarp contains only 3-9 wt%, d.b. oil. The cake obtained after the extraction of oil from the endosperm was mixed with the endocarp (20 wt% cake and 80 wt% endocarp) and used as feed (50 mg/larva/d) for the cultivation of Hermetia illucens larvae in a rearing container. The feed contains 39.2 wt%, d.b. hemicellulose, 10.9 wt%, d.b. cellulose and 29.9 wt%, d.b. lignin and 0.2 wt%, d.b. ash. The protein content of the feed was 19.1 wt%, d.b. A prepupal dry weight of approximately 50 3 mg/larvae was obtained after 12 d of treatment with an estimated productivity of 10.2 kgprepupae/m container.d. The estimated efficiency of black solider fly larvae in converting digested food was 21.6% with an assimilation efficiency of 27.7%. -

49 Some Malaysian Phytogeographical Problems
49 SOME MALAYSIAN PHYTOGEOGRAPHICAL PROBLEMS. B y E. D . MERRILL, Professor of Botany , Harvard University. Perhaps no part of the l\·orld is more intriguing from the standpoint of phytogeography than is the great equatorial archi pelago lying bet\Yeen southern Asia and Australia. :Malaysia is by far the largest island gronp in the world, lies ,,·holly \Yithin the humid tropics, has great diversity of altitudes np to nearly five thousand metres, and enjoys uniformly high low altitude temperatures, and, except in liibited regions, an abundant rainfall. Almost continuous precipitation occurs over large sections, accompanied by relatively high humidity; other large areas are characterized by alternating wet and dry seasons. These factors, combined with the characters of the soils, the topography, and the position of mountain masses in relation to prevaili ng winds provide optimum conditions for plant grm,·th, and the net result is a flora of tremendous richness and exuberance. The differentia tion of species has perhaps been in part favot·ed by the geoloo·ic development of the region, and its more or less insular character over long periods of time. Under present conditions contiguous parts of the same island may present rather strikingly different floras, \Yhile certain islands separated from each other by onl~· relatively narrow anns of the sea may have very strikingly different vegetative and floristic aspects. Some years ago on the basis of a study of endemism of thos~ parts of Malaysia blessed with published floras or compiled enumerations, such as the Malay Peninsula, Java, Borneo, and the Philippines, I concluded that when the flora was approxi mately known, that in the Pteridophytes and the Spermatophytes combined its total "·ould approximate to 45,ooo species.