Ancistrocladaceae
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Toward a Resolution of Campanulid Phylogeny, with Special Reference to the Placement of Dipsacales
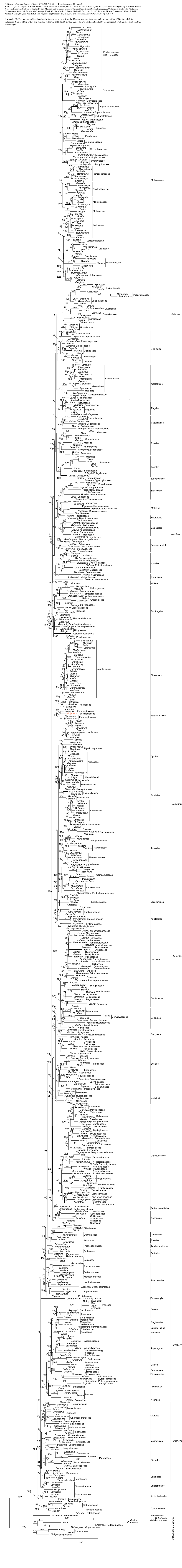
TAXON 57 (1) • February 2008: 53–65 Winkworth & al. • Campanulid phylogeny MOLECULAR PHYLOGENETICS Toward a resolution of Campanulid phylogeny, with special reference to the placement of Dipsacales Richard C. Winkworth1,2, Johannes Lundberg3 & Michael J. Donoghue4 1 Departamento de Botânica, Instituto de Biociências, Universidade de São Paulo, Caixa Postal 11461–CEP 05422-970, São Paulo, SP, Brazil. [email protected] (author for correspondence) 2 Current address: School of Biology, Chemistry, and Environmental Sciences, University of the South Pacific, Private Bag, Laucala Campus, Suva, Fiji 3 Department of Phanerogamic Botany, The Swedish Museum of Natural History, Box 50007, 104 05 Stockholm, Sweden 4 Department of Ecology & Evolutionary Biology and Peabody Museum of Natural History, Yale University, P.O. Box 208106, New Haven, Connecticut 06520-8106, U.S.A. Broad-scale phylogenetic analyses of the angiosperms and of the Asteridae have failed to confidently resolve relationships among the major lineages of the campanulid Asteridae (i.e., the euasterid II of APG II, 2003). To address this problem we assembled presently available sequences for a core set of 50 taxa, representing the diver- sity of the four largest lineages (Apiales, Aquifoliales, Asterales, Dipsacales) as well as the smaller “unplaced” groups (e.g., Bruniaceae, Paracryphiaceae, Columelliaceae). We constructed four data matrices for phylogenetic analysis: a chloroplast coding matrix (atpB, matK, ndhF, rbcL), a chloroplast non-coding matrix (rps16 intron, trnT-F region, trnV-atpE IGS), a combined chloroplast dataset (all seven chloroplast regions), and a combined genome matrix (seven chloroplast regions plus 18S and 26S rDNA). Bayesian analyses of these datasets using mixed substitution models produced often well-resolved and supported trees. -

Calophyllum Inophyllum L
Calophyllum inophyllum L. Guttiferae poon, beach calophyllum LOCAL NAMES Bengali (sultanachampa,punnang,kathchampa); Burmese (ph’ông,ponnyet); English (oil nut tree,beauty leaf,Borneo mahogany,dilo oil tree,alexandrian laurel); Filipino (bitaog,palo maria); Hindi (surpunka,pinnai,undi,surpan,sultanachampa,polanga); Javanese (njamplung); Malay (bentagor bunga,penaga pudek,pegana laut); Sanskrit (punnaga,nagachampa); Sinhala (domba); Swahili (mtondoo,mtomondo); Tamil (punnai,punnagam,pinnay); Thai (saraphee neen,naowakan,krathing); Trade name (poon,beach calophyllum); Vietnamese (c[aa]y m[uf]u) Calophyllum inophyllum leaves and fruit (Zhou Guangyi) BOTANIC DESCRIPTION Calophyllum inophyllum is a medium-sized tree up to 25 m tall, sometimes as large as 35 m, with sticky latex either clear or opaque and white, cream or yellow; bole usually twisted or leaning, up to 150 cm in diameter, without buttresses. Outer bark often with characteristic diamond to boat- shaped fissures becoming confluent with age, smooth, often with a yellowish or ochre tint, inner bark usually thick, soft, firm, fibrous and laminated, pink to red, darkening to brownish on exposure. Crown evenly conical to narrowly hemispherical; twigs 4-angled and rounded, with plump terminal buds 4-9 mm long. Shade tree in park (Rafael T. Cadiz) Leaves elliptical, thick, smooth and polished, ovate, obovate or oblong (min. 5.5) 8-20 (max. 23) cm long, rounded to cuneate at base, rounded, retuse or subacute at apex with latex canals that are usually less prominent; stipules absent. Inflorescence axillary, racemose, usually unbranched but occasionally with 3-flowered branches, 5-15 (max. 30)-flowered. Flowers usually bisexual but sometimes functionally unisexual, sweetly scented, with perianth of 8 (max. -

Lemur News 7 (2002).Pdf
Lemur News Vol. 7, 2002 Page 1 Conservation International’s President EDITORIAL Awarded Brazil’s Highest Honor In recognition of his years of conservation work in Brazil, CI President Russell Mittermeier was awarded the National Are you in favor of conservation? Do you know how conser- Order of the Southern Cross by the Brazilian government. vation is viewed by the academic world? I raise these ques- Dr. Mittermeier received the award on August 29, 2001 at tions because they are central to current issues facing pri- the Brazilian Ambassador's residence in Washington, DC. matology in general and prosimians specifically. The National Order of the Southern Cross was created in The Duke University Primate Center is in danger of being 1922 to recognize the merits of individuals who have helped closed because it is associated with conservation. An inter- to strengthen Brazil's relations with the international com- nal university review in 2001 stated that the Center was too munity. The award is the highest given to a foreign national focused on conservation and not enough on research. The re- for service in Brazil. viewers were all researchers from the "hard" sciences, but For the past three decades, Mittermeier has been a leader in they perceived conservation to be a negative. The Duke ad- promoting biodiversity conservation in Brazil and has con- ministration had similar views and wanted more emphasis ducted numerous studies on primates and other fauna in the on research and less on conservation. The new Director has country. During his time with the World Wildlife Fund three years to make that happen. -

Calophyllum Inophyllum (Kamani) Clusiaceae (Syn
April 2006 Species Profiles for Pacific Island Agroforestry ver. 2.1 www.traditionaltree.org Calophyllum inophyllum (kamani) Clusiaceae (syn. Guttiferae) (mangosteen family) Alexandrian laurel, beach mahogany, beauty leaf, poon, oil nut tree (English); beach calophyllum (Papua New Guinea), biyuch (Yap); btaches (Palau); daog, daok (Guam, N. Marianas); dilo (Fiji); eet (Kosrae); feta‘u (Tonga); fetau (Samoa); isou (Pohnpei); kamani, kamanu (Hawai‘i); lueg (Marshalls); rakich (Chuuk); tamanu (Cook Islands, Society Islands, Marquesas); te itai (Kiribati) J. B. Friday and Dana Okano photo: J. B. Friday B. J. photo: Kamani trees are most commonly seen along the shoreline (Hilo, Hawai‘i). IN BRIEF Growth rate May initially grow up to 1 m (3.3 ft) in height Distribution Widely dispersed throughout the tropics, in- per year on good sites, although usually much more slowly. cluding the Hawaiian and other Pacific islands. Main agroforestry uses Mixed-species woodlot, wind- break, homegarden. Size Typically 8–20 m (25–65 ft) tall at maturity. Main products Timber, seed oil. Habitat Strand or low-elevation riverine, 0–200 m (660 ft) Yields No timber yield data available; 100 kg (220 lb) in Hawai‘i, up to 800 m (2000 ft) at the equator; mean an- nuts/tree/yr yielding 5 kg (11 lb) oil. nual temperatures 18–33°C (64–91°F); annual rainfall 1000– Intercropping Casts a heavy shade, so not suitable as an 5000 mm (40–200 in). overstory tree; has been grown successfully in mixed-species Vegetation Occurs on beach and in coastal forests. timber stands. Soils Grows best in sandy, well drained soils. -

Calophyllum Inophyllum Beauty Leaf1 Edward F
Fact Sheet ST-115 November 1993 Calophyllum inophyllum Beauty Leaf1 Edward F. Gilman and Dennis G. Watson2 INTRODUCTION This upright, pyramidal, densely foliated evergreen tree can reach 60 feet in height in the forest with a 30 to 40-foot spread, but is generally much smaller because it grows slowly (Fig. 1). This is an asset in tropical landscapes, where many other plants grow so fast. Greenish, showy, 3/4-inch, very fragrant flowers are produced on eight-inch racemes in the summer. The round, yellow, 1.5-inch-wide fruit contains a single seed with a nutlike kernel that may be poisonous. The seven-inch-long, glossy, dark green, stiff, leathery leaves have numerous, distinct parallel veins at right angles to the midrib. The trunk has light grey, shallowly-ridged bark, and the wood is valued for boat building and cabinet work. GENERAL INFORMATION Scientific name: Calophyllum inophyllum Figure 1. Middle-aged Beauty Leaf. Pronunciation: kal-oh-FILL-um EYE-no-fill-um Common name(s): Beauty Leaf where air pollution, poor drainage, compacted soil, Family: Clusiaceae and/or drought are common USDA hardiness zones: 10B through 11 (Fig. 2) Availability: grown in small quantities by a small Origin: not native to North America number of nurseries Uses: container or above-ground planter; espalier; hedge; large parking lot islands (> 200 square feet in DESCRIPTION size); wide tree lawns (>6 feet wide); medium-sized parking lot islands (100-200 square feet in size); Height: 35 to 50 feet medium-sized tree lawns (4-6 feet wide); Spread: 30 to 50 feet recommended for buffer strips around parking lots or Crown uniformity: irregular outline or silhouette for median strip plantings in the highway; near a deck Crown shape: round; pyramidal or patio; reclamation plant; screen; shade tree; Crown density: dense specimen; sidewalk cutout (tree pit); residential street Growth rate: medium tree; tree has been successfully grown in urban areas 1. -

Creation and Carnivory in the Pitcher Plants of Nepenthaceae and Sarraceniaceae
OPEN ACCESS JCTS Article SERIES B Creation and Carnivory in the Pitcher Plants of Nepenthaceae and Sarraceniaceae R.W. Sanders and T.C. Wood Core Academy of Science, Dayton, TN Abstract The morphological adaptations of carnivorous plants and taxonomic distributions of those adaptations are reviewed, as are the conflicting classifications of the plants based on the adaptations, reproductive morphology, and DNA sequences. To begin developing a creationist understanding of the origin of plant carnivory, we here focus specifically on pitcher plants of Nepenthaceae and Sarraceniaceae because their popularity as cultivated curiosities has generated a literature resource amenable to baraminological analysis. Hybridization records were augmented by total nucleotide differences to assess species similarities. Nonhybridizing species falling within the molecular range of hybridizing species were included in the monobaramin of the hybridizing species. The combined data support each of the three genera of the Sarraceniaceae as a monobaramin, but the three could not be combined into a larger monobaramin. With the Nepenthaceae, the data unequivocally place 73% of the species in a single monobaramin, strongly suggesting the whole genus (and, thus, family) is a monobaramin. The lack of variation in the carnivorous habit provides no evidence for the intrabaraminic origin of carnivory from non-carnivorous plants. An array of fascinating symbiotic relationships of pitchers in some species with unusual bacteria, insects, and vertebrates are known and suggest the origin of carnivory from benign functions of the adaptive structures. However, these symbioses still do not account for the apparent complex design for carnivory characteristic of all species in the two families. Editor: J.W. -

Early Evolution of the Angiosperm Clade Asteraceae in the Cretaceous of Antarctica
Early evolution of the angiosperm clade Asteraceae in the Cretaceous of Antarctica Viviana D. Barredaa,1,2, Luis Palazzesia,b,1, Maria C. Telleríac, Eduardo B. Oliverod, J. Ian Rainee, and Félix Forestb aDivisión Paleobotánica, Museo Argentino de Ciencias Naturales “Bernardino Rivadavia,” Consejo Nacional de Investigaciones Cientificas y Técnicas, Buenos Aires C1405DJR, Argentina; bJodrell Laboratory, Royal Botanic Gardens, Kew, Richmond, Surrey TW9 3DS, United Kingdom; cLaboratorio de Sistemática y Biología Evolutiva, Museo de La Plata, La Plata B1900FWA, Argentina; dCentro Austral de Investigaciones Científicas, Consejo Nacional de Investigaciones Cientificas y Técnicas, 9410 Ushuaia, Tierra del Fuego, Argentina; and eDepartment of Palaeontology, GNS Science, Lower Hutt 5040, New Zealand Edited by Michael J. Donoghue, Yale University, New Haven, CT, and approved July 15, 2015 (received for review December 10, 2014) The Asteraceae (sunflowers and daisies) are the most diverse Here we report fossil pollen evidence from exposed Campanian/ family of flowering plants. Despite their prominent role in extant Maastrichtian sediments from the Antarctic Peninsula (Fig. 1, Fig. S1, terrestrial ecosystems, the early evolutionary history of this family and SI Materials and Methods, Fossiliferous Localities)(7)thatradi- remains poorly understood. Here we report the discovery of a cally changes our understanding of the early evolution of Asteraceae. number of fossil pollen grains preserved in dinosaur-bearing deposits from the Late Cretaceous of Antarctica that drastically pushes back Results and Discussion the timing of assumed origin of the family. Reliably dated to ∼76–66 The pollen grains reported here and discovered in the Late Cre- Mya, these specimens are about 20 million years older than previ- taceous of Antarctica are tricolporate, microechinate, with long ously known records for the Asteraceae. -

ORNAMENTAL GARDEN PLANTS of the GUIANAS: an Historical Perspective of Selected Garden Plants from Guyana, Surinam and French Guiana
f ORNAMENTAL GARDEN PLANTS OF THE GUIANAS: An Historical Perspective of Selected Garden Plants from Guyana, Surinam and French Guiana Vf•-L - - •• -> 3H. .. h’ - — - ' - - V ' " " - 1« 7-. .. -JZ = IS^ X : TST~ .isf *“**2-rt * * , ' . / * 1 f f r m f l r l. Robert A. DeFilipps D e p a r t m e n t o f B o t a n y Smithsonian Institution, Washington, D.C. \ 1 9 9 2 ORNAMENTAL GARDEN PLANTS OF THE GUIANAS Table of Contents I. Map of the Guianas II. Introduction 1 III. Basic Bibliography 14 IV. Acknowledgements 17 V. Maps of Guyana, Surinam and French Guiana VI. Ornamental Garden Plants of the Guianas Gymnosperms 19 Dicotyledons 24 Monocotyledons 205 VII. Title Page, Maps and Plates Credits 319 VIII. Illustration Credits 321 IX. Common Names Index 345 X. Scientific Names Index 353 XI. Endpiece ORNAMENTAL GARDEN PLANTS OF THE GUIANAS Introduction I. Historical Setting of the Guianan Plant Heritage The Guianas are embedded high in the green shoulder of northern South America, an area once known as the "Wild Coast". They are the only non-Latin American countries in South America, and are situated just north of the Equator in a configuration with the Amazon River of Brazil to the south and the Orinoco River of Venezuela to the west. The three Guianas comprise, from west to east, the countries of Guyana (area: 83,000 square miles; capital: Georgetown), Surinam (area: 63, 037 square miles; capital: Paramaribo) and French Guiana (area: 34, 740 square miles; capital: Cayenne). Perhaps the earliest physical contact between Europeans and the present-day Guianas occurred in 1500 when the Spanish navigator Vincente Yanez Pinzon, after discovering the Amazon River, sailed northwest and entered the Oyapock River, which is now the eastern boundary of French Guiana. -

Croton Production and Use1 Robert H
ENH878 Croton Production and Use1 Robert H. Stamps and Lance S. Osborne2 FAMILY: Euphorbiaceae GENUS: Codiaeum SPECIFIC EPITHET: variegatum CULTIVARS: ‘Banana’, ‘Gold Dust’, ‘Mammy’, ‘Norma’, ‘Petra’, ‘Sunny Star’ and many others. Crotons have been popular in tropical gardens for centuries. Crotons grow into shrubs and small trees in their native habitats of India, Malaysia, and some of the South Pacific islands. Few other plants can surpass them in both foliage color and leaf shape variation. Leaf colors range from reds, oranges and yellows to green with all combinations of variegated colors. Leaf shapes vary from broad and elliptical to narrow and almost linear. Leaf blades range from flat to cork-screw-shaped. Since some cultivars are tolerant of interior environments, crotons have also become very popular as interior potted foliage plants. One additional point, often overlooked, is that foliage of crotons Figure 1. Crotons are useful for adding color to floral arrangements, is excellent material for use in floral arrangements. Both landscapes, and interiorscapes. individual leaves and entire branches can be used in floral Credits: Robert Stamps, UF/IFAS designs. 1. This document is ENH878, one of a series of the Environmental Horticulture Department, UF/IFAS Extension. Original publication date December 2002. Revised Revised May 2009 and March 2019. Visit the EDIS website at https://edis.ifas.ufl.edu for the currently supported version of this publication. 2. Robert H. Stamps, professor of Environmental Horticulture and Extension Cut Foliage Specialist; and Lance S. Osborne, professor of Entomology; UF/ IFAS Mid-Florida Research and Education Center, Apopka, FL. The use of trade names in this publication is solely for the purpose of providing specific information. -

Deletion of Cephalotus Follicularis from Appendix II
Prop. 11.6 CONVENTION ON INTERNATIONAL TRADE IN ENDANGERED SPECIES OF WILD FAUNA AND FLORA A. PROPOSAL Deletion of Cephalotus follicularis from Appendix II. B. PROPONENT Commonwealth of Australia (Environment Australia) C. SUPPORTING STATEMENT The monotypic genus Cephalotus is an insectivorous plant endemic to south western Australia. It occurs on wetland margins throughout the southwest corner of Western Australia. This portion of Western Australia has a high rainfall and as a result there are extensive areas of suitable habitat, especially on the south coastal plain. Within its range are large areas of government owned forests, National Parks and other reserves where the species is common and is likely to occur in vast numbers. The species is easily propagated from small segments of rhizomes and, as a result, it is commonly traded and is widely cultivated. Morphological variation in wild populations is not evident. As the species is easily propagated, it is unlikely that cultivated stocks are derived from wild collections. Cephalotus follicularis has been identified by the CITES Plants Committee under the Ten Year Review as a candidate for deletion from Appendix II as there has been no recorded trade in wild taken specimens since the species was listed. The proposal received full endorsement of the 5th meeting of the CITES Plants Committee in Mexico, May 1994. 1. Taxonomy 1.1. Class Magnoliatae 1.2. Order Rosales 1.3. Family Cephalotaceae 1.4. Genus/species Cephalotus follicularis Labillardière 1806 1.5. Common name Western Australian pitcher plant; Albany pitcher plant 2. Biological data 2.1. Distribution The area of distribution ranges over 400 km from NW to SE and corresponds with the meso- mediterranean climate of the extreme south western part of Australia. -

Widespread Paleopolyploidy, Gene Tree Conflict, and Recalcitrant Relationships Among the 3 Carnivorous Caryophyllales1 4 5 Joseph F
bioRxiv preprint doi: https://doi.org/10.1101/115741; this version posted March 10, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. 1 2 Widespread paleopolyploidy, gene tree conflict, and recalcitrant relationships among the 3 carnivorous Caryophyllales1 4 5 Joseph F. Walker*,2, Ya Yang2,5, Michael J. Moore3, Jessica Mikenas3, Alfonso Timoneda4, Samuel F. 6 Brockington4 and Stephen A. Smith*,2 7 8 2Department of Ecology & Evolutionary Biology, University of Michigan, 830 North University Avenue, 9 Ann Arbor, MI 48109-1048, USA 10 3Department of Biology, Oberlin College, Science Center K111, 119 Woodland St., Oberlin, Ohio 44074- 11 1097 USA 12 4Department of Plant Sciences, University of Cambridge, Cambridge CB2 3EA, United Kingdom 13 5 Department of Plant Biology, University of Minnesota-Twin Cities. 1445 Gortner Avenue, St. Paul, MN 14 55108 15 CORRESPONDING AUTHORS: Joseph F. Walker; [email protected] and Stephen A. Smith; 16 [email protected] 17 18 1Manuscript received ____; revision accepted ______. bioRxiv preprint doi: https://doi.org/10.1101/115741; this version posted March 10, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. 19 ABSTRACT 20 • The carnivorous members of the large, hyperdiverse Caryophyllales (e.g. -

Giant Cephalotus of Unknown Origins
Giant Cephalotus of unknown origins Dick Chan • P.O. Box 2252 • Pasadena • California 91102 • USA • [email protected] Introduction I have been growing Cephalotus follicularis for over 20 years. Initially, I was obsessed with grow- ing specimen-type Cephalotus of different clones and to prove once-and-for-all that this was not a difficult plant to grow. Countless plants have met their demise as I experimented with various meth- ods of cultivation. For those that have survived and flourished, I noticed one plant in particular that grew larger, more vigorous, and had a different pitcher/leaf morphology than Cephalotus ‘Hummer’s Giant’ and the typical Cephalotus. However, I do not believe this plant to be just a better-grown speci- men of ‘Hummer’s Giant’. Through the years, I have given and sold this plant to individuals calling it the “Bubble Giant”, however, I have not received nor heard any feedback as to the well-being of those plants. So, for those reading this article and have received this plant from me, I would appreciate seeing some photos. For the remainder of this article, this plant will be referred to as the “unknown”. Origins During my initial spark-of-entry into the hobby, I started collecting Cephalotus cuttings, plants, stems, and leaves from anyone who had the plant and was willing to give or sell a piece to me. Be- cause of that activity, this plant is of an unknown origin because of the feverish pace by which I went about amassing what I had hoped would become a genetically diverse collection of plants.