Podarcis Erhardii.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chania : Explore & Experience
INDEX INDEX .......................................................................................................................................... 1 THE BYZANTINE WALL OF CHANIA ............................................................................................. 3 THE EGYPTIAN LIGHTHOUSE ...................................................................................................... 4 GIALI TZAMISI ............................................................................................................................. 5 VENETIAN NEORIA ...................................................................................................................... 6 FIRKA FORTRESS ......................................................................................................................... 7 CENTER OF MEDITERRANEAN ARCHITECTURE (GRAND ARSENAL)............................................ 8 ANCIENT KYDONIA (PROTO-MINOAN SETTLEMENT OF KASTELI) .............................................. 9 ANCIENT APTERA ......................................................................................................................10 ENTRANCE OF THE RENIER MANSION ......................................................................................11 GATE AND RAMPART SABBIONARA .........................................................................................12 THE MINARET OF AGIOS NIKOLAOS .........................................................................................13 THE GRAVES OF VENIZELOS FAMILY ........................................................................................14 -

Greek Gazetteer � Vol
! GREEK GAZETTEER ! VOL. 2, Part Ia, Part Ib ! ! ! ! ! ! ! By Lica H. Catsakis (Bywater) ! Salt Lake City, Utah 2000 ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! Published by Lica H. Catsakis 71 S. Chalon Dr. St. George, !Utah 84770 Copyright © 2000 by Lica H. Catsakis (Bywater). All rights reserved. First edition of vol.2 published 2000 Printed in the United States of America ! ! ! ! "ii! ! TABLE OF CONTENTS ! ! Page VOLUME 1 Acknowledgment .......................................................................................................... ii Introduction ...................................................................................................................iii Romanization Chart ...................................................................................................... vi Explanation of Abbreviations and Greek Terms ...........................................................viii Eparhia (District) and Capital City ...............................................................................x Nomos (County) and Capital City ................................................................................ xiv !Mitropolis (Diocese) and Seat of Diocese .................................................................... xvi Part I Map of Greece ...................................................................................................PART 1, p 2 Administrative Division of Greece ...................................................................PART 1, p 3 -

Merging Giants John Bessis Country Manager Greece and Cyprus, Allergan Aesthetics Pascal Apostolides Managing Director, Abbvie in Greece
AbbVie_ad_208x280tel.pdf 1 27/4/20 3:48 PM JULY-AUGUST 2020 THE MAGAZINE OF THE AMERICAN-HELLENIC CHAMBER OF COMMERCE www.amcham.gr C M Y CM MY CY CMY K MERGING GIANTS JOHN BESSIS COUNTRY MANAGER GREECE AND CYPRUS, ALLERGAN AESTHETICS PASCAL APOSTOLIDES MANAGING DIRECTOR, ABBVIE IN GREECE THOUGHT LEADERS KYRIAKOS PIERRAKAKIS IN AN ALLIANCE FOR SMEs AND STARTUPS DIGITAL REVOLUTION IOANNA LYTRIVI TALKS ABOUT EDUCATION AND SPEARHEADING EMPLOYMENT IN POST-COVID-19 GREECE GROWTH GREEK INVESTMENT FORUM – NEW YORK Business-partners-option-2.pdf 1 9/3/2020 15:01:24 Business-partners-option-2.pdf 1 9/3/2020 15:01:24 We Create Workplaces that are Destinations for the Human Interaction We Create Workplaces that are Destinations for the Human Interaction C C M M Y Y CM CM MY MY CY CY CMY CMY K K It takes more than just ergonomic furniture and a tness center to achieve It takes more than just ergonomic furniture and a tness center to achieve Wellbeing at work. It’s about creating a culture of wellbeing Wellbeing at work. It’s about creating a culture of wellbeing where people can Move, Think and Feel Better. where people can Move, Think and Feel Better. 1 Kisias Avenue, Marousi | T: 211 212 0820 | E: [email protected] | W: www.ekahellas.com | ekahellas 1 Kisias Avenue, Marousi | T: 211 212 0820 | E: [email protected] | W: www.ekahellas.com | ekahellas CONTENTS 24 16 35 Pascal Apostolides and John Bessis talk Kyriakos Pierrakakis talks about the EOPPEP CEO Ioanna Lytrivi talks to about AbbVie’s landmark acquisition of changes and challenges brought -

Nature Park of Sitia Is on the Easternmost Edge of Crete, in the Municipality of Sitia
This publication was designed by the Natural History Museum of Crete for the Municipal- ity of Sitia, due to the implementation of the action 2.3.1 “Development of an Ecotouristic guide” of the project “Geotourism and local development (GEOTOPIA)”, funded 80% by the European Union and by 20% by national funds from Greece and Cyprus, through the Greece-Cyprus 2007 - 2013 cross-border cooperation programme. “GEOTOPIA” refers to the collaboration of two mountainous and insular areas, the Munici- pality of Sitia in Crete and the mountain Troodos in Cyprus, which are characterized by their wealthy natural, geological and cultural environment, by underdevelopment and depopu- lation of the hinterland, and by the depreciation of their landscape, aiming to promote their natural and cultural environment, to develop geotouristic activities and finally, establish a geopark. Museum Scientific Coordinator: Dr Charalampos Fassoulas Authors: Fassoulas C. – Dr geologist, Staridas S. – Msc geologist, Perakis N. – environmentalist, Mavroudi N. – archaeologist, Trichas A. – Dr biologist, Avramakis M. – botanist, Perakis V. – botanist, Mavrokosta C. – speleologist. Map design: Staridas S. Graphics design: Harkoutsis G. Text compilation: Dr Fassoulas C. Text correction: Mavroudi N. Translation in English: Interpretation and Translation Center. Jeni Kantarti Loutsa & collaborators, Thessaloniki Copyright: Natural History Museum of Crete / University of Crete, Sitia Nature Park Copyright of pictures and illustrations: Natural History Museum of Crete / Uni. of -

Crete (Chapter)
Greek Islands Crete (Chapter) Edition 7th Edition, March 2012 Pages 56 Page Range 256-311 PDF Coverage includes: Central Crete, Iraklio, Cretaquarium, Knossos, Arhanes, Zaros, Matala, Rethymno, Moni Arkadiou, Anogia, Mt Psiloritis, Spili, Plakias & around, Beaches Between Plakias & Agia Galini, Agia Galini, Western Crete, Hania & around, Samaria Gorge, Hora Sfakion & around, Frangokastello, Anopoli & Inner Sfakia, Sougia, Paleohora, Elafonisi, Gavdos Island, Kissamos-Kastelli & around, Eastern Crete, Lasithi Plateau, Agios Nikolaos & around, Mohlos, Sitia & around, Kato Zakros & Ancient Zakros, and Ierapetra & around. Useful Links: Having trouble viewing your file? Head to Lonely Planet Troubleshooting. Need more assistance? Head to the Help and Support page. Want to find more chapters? Head back to the Lonely Planet Shop. Want to hear fellow travellers’ tips and experiences? Lonely Planet’s Thorntree Community is waiting for you! © Lonely Planet Publications Pty Ltd. To make it easier for you to use, access to this chapter is not digitally restricted. In return, we think it’s fair to ask you to use it for personal, non-commercial purposes only. In other words, please don’t upload this chapter to a peer-to-peer site, mass email it to everyone you know, or resell it. See the terms and conditions on our site for a longer way of saying the above - ‘Do the right thing with our content. ©Lonely Planet Publications Pty Ltd Crete Why Go? Iraklio ............................ 261 Crete (Κρήτη) is in many respects the culmination of the Knossos ........................268 Greek experience. Nature here has been as prolifi c as Picas- Rethymno ..................... 274 so in his prime, creating a dramatic quilt of big-shouldered Anogia ......................... -

Crete 0 10 Miles
e# 0 20 km Crete 0 10 miles 36º N Archaeological Museum Marvel at treasures from ELEVATION ancient worlds 2000m 1500m Hania’s Old Town Rethymno’s Old Quarter Stroll the charming Experience the romance of 1000m Iraklio Wine Country Spinalonga Island 500m Venetian Harbour a Renaissance town SEA OF CRETE Sip Crete’s top Visit the leper colony turned 0 vintages tourist attraction Cape Spatha ä# Diktynna Moni Arkadiou Palace of Knossos Cape Rodopos Meditate on beauty and Walk in the footsteps of Vouxa Peninsula Gulf of the Minoans Gramvousa Moni Hania Stavros Moni Iannou Eremiti tragic history Islets Bay of #\ ä# Gonias Ü# Moni Governotou Bay of Kalathas Ü# GramvousaKissamos Ü# Vaï Beach Peninsula #\ Akrotiri Moni Agias Triadas Kissamos Kolymbari Peninsula #– Kick back under palm Falasarna #\ #\ #\ (Kastelli) Souda Bay trees #\ Platanias Hania #\ #\ #\ Voukolies Souda Cape Drapano Platanos ä# Cape Polyrrina Drapano Panormo Stavros Dia HANIA Peninsula #\ #\ Bali Almyros Bay #\ Rethymno Iraklio 33 Vryses #\ #\ Cape Agios Samaria Perama Moni Agia Omalos #\ Bay Iraklio Ioannis Cape #\ Gorge Georgioupolis Sideros Irini #\ Margarites #\ Axos ^# #– Hrysoskalitissas #\ National #\ Hersonisos Park Lake Episkopi Moni Ü# ä# Eleftherna ä# #\ Spinalonga Island Ü# Kandanos Lefka Ori ä## Malia R #\ Knossos #\ ä# #÷ Kournas#\ Arkadiou Sfendoni # Elounda (2453m) Argyroupoli #\ Kolokytha Moni #\ #\ Elafonisi # Samaria Cave Anogia Malia #\ Vaï 3Gorg3e Neapoli Peninsula Toplou #\ RETHYMNO Imbros Mt Psiloritis #\ Ü# #\ Sougia #\ Hora # Spili R Arhanes Ancient -

Booklet Concerning Ecosystem Services of Coastal Areas In
NATURA 2000 Network Ecosystem of Coastal Services Areas of Crete INFORMATION GUIDE This publication was implemented by the University of Crete - Natural History Museum of Crete (NHMC) in the framework of the LIFE Natura 2000 Value Crete project: “The ecological services, social benefits and economic value of the Ecosystem Services in Natura 2000 sites in Crete” (LIFE13 INF/GR/000188). The project is co-financed by the European Commission/DG Environment at a percentage of 50% and was also co-financed by the Ministry of Environment and Energy (MEEN), the Green Fund and the A. G. Leventis Foundation. Associated beneficiaries are the Decentralized Administration Authority of Crete – Directorate of Coordination and Supervision of Forests and the Hellenic Ornithological Society (HOS). UNIVERSITY OF CRETE – NATURAL HISTORY MUSEUM OF CRETE Knossos Avenue Premises GR714 09 Heraklion, Crete Copyright © University of Crete - Natural History Museum of Crete Authors: Tania Ploumi, Elisavet Georgopoulou, Niki Kyriakopoulou Scientific editing: University of Crete - Natural History Museum of Crete Publication coordination: Michalis Probonas Editing - Correction of texts: Panagiotis Georgiakakis, Popi Baxevani Graphics editing: Giannis Harkoutsis Maps editing: Elisavet Georgopoulou English translation: Elisavet Georgopoulou Suggested Reference: Tania Ploumi, Elisavet Georgopoulou, Niki Kyriakopoulou. 2018. Information Guide for Ecosystem Services of the NATURA 2000 Network sites in the Coastal Areas of Crete. University of Crete - Natural History Museum of Crete, Heraklion, pp. 56. Printing: KAMPILI S.A. HERAKLION 2018 COMPLIMENTARY COPY ISBN: 978-960-367-044-5 The partial or total reproduction, use or re-printing is forbidden without the written permission of the University of Crete - Natural History Museum of Crete. -

Introduction Acknowledgements
10 11 Acknowledgements Introduction General geography of Greece Greece is a relatively small country, and with a surface area of 132,000 km2 it is only half as big as the UK. Encompassed, however, in this modest area, is a great diversity of habitats, exceeding many European countries of much larger size. For example, one can encounter in Epirus alpine areas complete with lush conifer forests, dramatic peaks and extensive snowfields that physiographically resemble Switzerland. On the other hand, some regions of the southern Aegean are closer to Africa than to Athens, and their climate and habitats reflect this proximity. Southeastern Crete for example, con- tains one of the few true European deserts, an area closely resembling certain hamma- da regions of the Middle East. Greece is a country of mountains and islands. The Pindos range, an extension of the Dinaric Alps, forms the backbone of peninsular Greece. A number of smaller mountains originate as spurs from this block, although some, including Mount Olympus, the highest mountain in Greece (2,917 m elevation) arise in relative isola- tion. A second major mountain block, the Rhodopes, located in Thrace, runs in a roughly east-west direction separating Greece and Bulgaria. The Peloponnese, a small- er peninsula in the south, is as mountainous as the mainland and encompasses several peaks exceeding 2,000 m in elevation. With the exception of a few large flat regions located mostly in Thessaly and Thrace, the country lacks extensive plains. Typically the mountains drop rather steeply into the sea and are generally flanked only by narrow coastal plains. -
Top 10 Crete
EYEWITNESS TRAVEL TOP10 CRETE N O ORO ID S U K B O OF M OR I EN 10 5 A UT LIKO MA 2 MA LI Best beaches K Agios E O S OUT PLATIA I Titos AGIOS I TOU ARI ADNI AS TITOS S T S 10 R IO IGI O Must-see museums & ancient sites AY F M I R A B E L O U Battle of Crete O B Loggia AN Museum S 10 O Venetian DHR K Spectacular areas of natural beauty HA M D ZID A K I U Walls IL DOU OG ATO O U S D EO HÍ D 10 K Best traditional tavernas D O Archaeological EDHALOU RA I APOUTIE Museum S THOU IDOMENEO N A 10 D Most exciting festivals 10 Liveliest bars & clubs 10 Best hotels for every budget 10 Most charming villages 10 Fascinating monasteries & churches 10 Insider tips for every visitor YOUR GUIDE TO 10THE 10 BEST OF EVERYTHING TOP 10 CRETE ROBIN GAULDIE EYEWITNESS TRAVEL Left Dolphin fresco, Knosos Right Rethymno harbour Contents Crete’s Top 10 Contents Ancient Knosos 8 Irakleio 12 Produced by Blue Island Publishing Reproduced by Colourscan, Singapore Printed Irakleio Archaeological and bound in China by Leo Paper Products Ltd First American Edition, 2003 Museum 14 11 12 13 14 10 9 8 7 6 5 4 3 2 1 Chania 18 Published in the United States by DK Publishing, 375 Hudson Street, Phaestos 20 New York, New York 10014 Reprinted with revisions Rethymno 22 2005, 2007, 2009, 2011 Gortys 24 Copyright 2003, 2011 © Dorling Kindersley Limited Samaria Gorge 26 All rights reserved. -
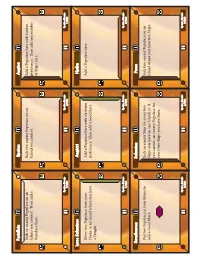
Print and Play
Psytalleia Spetses Aegina Sack an enemy Populace on an Sack two enemy Populace on an Add a Populace here with timber Island you control. Then add a Island you control. preference. Then add any number Populace there. of Ships here. Sparta - Bronze Sparta - Bronze Sparta - Bronze 7/220 4/220 1/220 Leros Salaminos Angistri Hydra Move two Populace from your Add a Populace here with electrum Add a Populace here. Home to an Island where you have preference. Then add a Good here. a Temple. Sparta - Bronze Sparta - Bronze Sparta - Bronze 8/220 5/220 2/220 Revythoussa Salamina Poros Pay two Populace at your Home to Sack an enemy Ship for every two Sack two enemy Populace on an add a Good there. Ships you have on one Island, or if Island where you have two Ships. you cannot, an enemy Populace for every two Ships you have there. Sparta - Bronze Sparta - Bronze Sparta - Bronze 9/220 6/220 3/220 Plateia Spetsopoula Moni Aiginas Sack an enemy Populace on every Add a Populace here. Then pay Add a Populace here with bronze Island where you have a Populace a Good here to add a Populace at preference. Then add another except your Home. each Island with a Good. Populace here. Sparta - Bronze Sparta - Bronze Sparta - Bronze 16/220 13/220 10/220 Bisti Petrokaravo Dokos Move one or more of your Populace Move three Populace from Home to Sack an enemy Populace on each on one Ship to any other Island. an Island where you have a Temple Island where you have a Ship. -

Ecotouristic Guide in Crete
LIFE – ENVIRONMENT «MEDITERRANEAN RESERVOIRS AND WETLANDS: A DEMOSTRATION OF MULTIPLE-OBJECTIVE MANAGEMENT IN THE ISLAND OF CRETE» LIFE00ENV/GR/000685 ECOTOURISTIC GUIDE IN CRETE INDEX Introduction Map of Cretan wetlands 1. Ierapetra harbor and Bramiana reservoir 2. Agios Nikolaos harbor and Almyros marsh 3. Elounda saltpans and Spinalonga peninsula 4. Malia marsh 5. Gouves lagoon and Aposelemis river 6. Κarteros river mouth 7. Αlmyros river – Heraklion power station 8. Thrapsano reservoir 9. Partira and Amourgelles reservoirs 10. Karavados & Skinias reservoir 11. Plakiotissa dam & Anapodaris river 12. Lake Digeni and Gergeri reservoir 13. Phaestos, Aghia Triada and Geropotamos 14. Aghia Galini and Platis river 15. Moni Preveli and Megalopotamos valley 16. Outfall and springs of Geropotamos - Rethymno 17. Petres river and gorge 18. Lake Kourna 19. Georgioupolis lake 20. Frangocastello 21. Souda bay – Vlites 22. Aghia lake 23. Keritis river mouth and beach 24. Tavronitis river 25. Falasarna ECOTOURISTIC GUIDE IN CRETE INTRODUCTION he island of Crete lies at the center of the eastern Mediterranean basin. The south most point Tof Europe is to be found on one of Crete’s satellite islands, Gavdos, with its south cape Tripiti stretching out into the Libyan Sea. Crete is particularly mountainous and has many high peaks, three of which are over 2.000 m. high. The summit of Idi (Psiloritis) rises up to 2.456 m. above sea level. Calcareous substrate that easily erodes has shaped many canyons and caves throughout the island. The seashore is full with bays and gulfs steep slopes, caves and many seastrands. There is a significant number of bigger or smaller islands surrounding Crete, the most important being: Pontikonisi, Grambousa, Dia, Psira, Paximada, Koufonisi, Hrisi, Paximadia, Gavdos, Gavdopoula and Elaphonisi. -

Kefalonia Island, Ionian
Argostoli 1 KEFALONIA It has been the capital town of the island since 1757. Argostoli is a modern town that keeps KEFALONIA many of the traditional architectural elements of the past. It has been built in an amphithea- 51 tre-like manner, overlooking Koutavos lagoon, a stopover area for many migratory birds. A large number of neoclassical buildings, large squares and churches grace the town. There is a remarkable diversity of cultural events as the town’s Philharmonic Band, choirs, and drama groups are quite active. Town promenade 47. Argostoli: the commemorative obelisk • To the central, spacious Vallianos Square, at Drapanos Bridge. where the statue of benefactor P. Vallianos vos lagoon is marvellous. stands surrounded by cafés, restaurants, and • To the Municipal Market, by the beach, bars. where the bust of local poet Nikos Kavvadias • To Napier’s Garden, named after British Lord stands. High Commissioner Sir Charles James Napier • To Drapanos stone bridge (also named De who, in the mid-19th century carried out many Bosset Bridge). Built by the British in 1813, it infrastructure works on the island. Though small is 900 metres long and links Argostoli with the in size, the garden has a large variety of tree opposite coast. Do visit the Municipal Cem- species. etery at nearby Drapanos village where there • To Rizospaston Street, bordered by palm are exquisite marble sculptures and monuments trees; this is the location of Risospaston Monu- created by famous artists. ment. • To the Archeological Museum, exhibiting • To Lithostroto, the town’s main street lined finds from various sites on the island, mostly with shops and haunts of the young.