Landscape and Vegetation of Southern Baltic Dune Systems
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

RESTORATION ACTION PLAN MARINA DUNES PRESERVE Marina, California
RESTORATION ACTION PLAN MARINA DUNES PRESERVE Marina, California Prepared for: Monterey Peninsula Regional Park District 4860 Carmel Valley Road Carmel, CA 93923 Prepared by: Burleson Consulting Inc. 1900 Garden Road, Suite 210 Monterey, CA 93940 March 2021 This page intentionally left blank Restoration Action Plan, Marina Dunes Preserve CONTENTS CONTENTS ..........................................................................................................................................i APPENDICES ...................................................................................................................................... ii ACRONYMS AND ABBREVIATIONS ..................................................................................................... iii 1. INTRODUCTION ...................................................................................................................... 1 1.1 Setting ........................................................................................................................................... 1 1.2 Purpose ......................................................................................................................................... 1 1.3 Approach ....................................................................................................................................... 2 2. UPDATED BEST MANAGEMENT PRACTICES .............................................................................. 3 2.1 Weed Eradication and Control ..................................................................................................... -

IREK Nord-Rügen
Wir auf Jasmund – Gemeinsam für Rügen! Integriertes Regionales Entwicklungskonzept (IREK) für die Gemeinden Breege, Glowe, Lohme und Sagard sowie die Stadt Sassnitz Landgesellschaft Mecklenburg-Vorpommern mbH Abteilung Stadt- und Regionalentwicklung Lindenallee 2a Telefon 03866 404-0 19067 Leezen Telefax 03866 404-490 lgmv.de E-Mail [email protected] Integriertes Regionales Entwicklungskonzept (IREK) für die Gemeinden Breege, Glowe, Lohme und Sagard sowie die Stadt Sassnitz Stand: 24.11.2020 Auftraggeber Gemeinde Lohme über das Amt Nord-Rügen Ernst-Thälmann-Straße 37 18551 Sagard Auftragnehmer Landgesellschaft Mecklenburg-Vorpommern mbH Abteilung Stadt- und Regionalentwicklung Lindenallee 2 a 19067 Leezen Bearbeitung Ute Franke, Ines Herrmann, Torsten Mehlhorn, Matti Skor Telefon 03866 404-0 Telefax 0385 404-490 E-Mail: [email protected] Internet: www.lgmv.de Hinweis Zugunsten einer besseren Lesbarkeit wird auf eine konsequent geschlechtergerechte Spra- che verzichtet. Dies ist rein stilistisch und nicht in einer Gesinnung begründet. Das meist im Plural gewählte, weil kürzere, generische Maskulinum steht ausdrücklich für alle Geschlech- ter. Die Verfasser bitten die Leserinnen und Leser um Verständnis. Integriertes Regionales Entwicklungskonzept Gemeinden Breege, Glowe, Lohme, Sagard und Stadt Sassnitz INHALTSVERZEICHNIS 1. Kurzfassung ............................................................................................................................... 4 2. Grundlagen und Vorbetrachtungen ................................................................................... -

Low Atmospheric Nitrogen Loads Lead to Grass Encroachment in Coastal Dunes, but Only on Acid Soils
Ecosystems (2009) 12: 1173–1188 DOI: 10.1007/s10021-009-9282-0 Ó 2009 The Author(s). This article is published with open access at Springerlink.com Low Atmospheric Nitrogen Loads Lead to Grass Encroachment in Coastal Dunes, but Only on Acid Soils Eva Remke,1,4* Emiel Brouwer,2 Annemieke Kooijman,3 Irmgard Blindow,1 and Jan G. M. Roelofs5 1Biological Station of Hiddensee, Ernst-Moritz-Arndt-University Greifswald, Biologenweg 15, 18565 Kloster, Germany; 2Research Center B-WARE B.V., Radboud University Nijmegen, Heyendaalseweg 135, 6525 AJ Nijmegen, The Netherlands; 3Institute of Biodi- versity and Ecosystem Dynamics, Physical Geography, University of Amsterdam, Nieuwe Achtergracht 166, 1018 WV Amsterdam, The Netherlands; 4Bargerveen Foundation, Department of Animal Ecology, Radboud University Nijmegen, Toernooiveld 1, 6525 ED Nijmegen, The Netherlands; 5Department of Aquatic Ecology and Environmental Biology, Radboud University Nijmegen, Hey- endaalseweg 135, 6525 AJ Nijmegen, The Netherlands ABSTRACT The impact of atmospheric N-deposition on suc- with elevated N-deposition, which may further cession from open sand to dry, lichen-rich, short stimulate Carex arenaria. Due to high growth plas- grassland, and tall grass vegetation dominated by ticity, efficient resource allocation and tolerance of Carex arenaria was surveyed in 19 coastal dune sites high metal concentrations, C. arenaria is a superior along the Baltic Sea. Coastal dunes with acid or competitor under these conditions and can start to slightly calcareous sand reacted differently to dominate the dune system. Carex-dominated vege- atmospheric wet deposition of 5–8 kg N ha-1 y-1. tation is species-poor. Even at the moderate N- Accelerated acidification, as well as increased loads in this study, foliose lichens, forbs and grasses growth of Carex and accumulation of organic mat- were reduced in short grass vegetation at acid sites. -
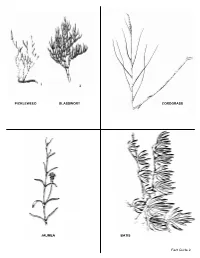
Plant Field Guide
2 PICKLEWEED GLASSWORT CORDGRASS JAUMEA BATIS Field Guide 9 PICKLEWEED Amaranth Family 3 kinds, 2 examples CORDGRASS Grass Family 1 Pickleweed Sarcocornia pacifica Spartina foliosa Glasswort Arthrocnemum subterminalis 2 HABITAT: Growns in the low marsh where the HABITAT: Found throughout the salt marsh. roots are continually bathed in ocean water. APPEARANCE: Stems look like a chain of small APPEARANCE: Look for a tall grass which is pickles. higher than the other plants in the salt marsh. REPRODUCTION: The flowers of all pickleweeds REPRODUCTION: All grasses are wind pollinated. are pollinated by the wind. The small flowers are Look for straw colored spikes of densely packed hard to see because they have no colorful petals flowers. Male flowers will have pollen and the female flowers will show graceful waving stigmas to ADAPTATION TO SALT: Pickleweeds are some of catch the pollen. the many marsh plants that use salt storage (they are accumulators). Also called succulents, these ADAPTATION TO SALT: All the salt marsh plants are swollen with the stored salty water. grasses are salt excreters using special pores to When the salt concentration becomes too high the push out droplets of salty water. Look on the grass cells will die. blades for salt crystals. See sea lavender. ECOLOGICAL RELATIONSHIPS: Frequently the ECOLOGICAL RELATIONSHIPS: Home for the most common plants in the marsh, they provide endangered bird, the Light-footed Clapper Rail. shelter and food for invertebrates. Belding’s A spider lives its whole life inside the blades. Savannah Sparrows build their nests in the Important food for grazing animals. glasswort. BATIS or SALTWORT Saltwort Family Batis maritima HABITAT: Most frequently found in the low marsh. -

Rostock Hbf - Stralsund Hbf - Bergen Auf Rügen � Ostseebad Binz 190
Kursbuch der Deutschen Bahn 2021 www.bahn.de/kursbuch Sassnitz Ostseebad Binz 190 ر Rostock Hbf - Stralsund Hbf - Bergen auf Rügen 190 VVW Verbundtarif Rostock - Gelbensande Zug RE 9 RE 9 RE 9 RE 9 RB 12 RE 10 RE 10 RE 9 ICE RE 9 RE 9 RB 12 ICE ICE 76351 76353 76355 76357 13227 76455 76455 76359 949 76397 76361 13231 1678 1678 f2. 76391 76393 76395 f f2. f2. f2. f2. 76399 f hy hy f2. f2. Ẅ f2. ẇ f2. ẅ Ẇ Ẉ ẅ Ẇ km km von Bonn Hbf Schwerin Hannover Hbf Hbf 45 9 ܥ ẚẍ 9 38 27 9 ܥ 00 9 11 8 ܥ ẙẕ 8 11 27 7 ܥ Rostock Hbf 181-185, 205 ẞẖ ݜ 4 54 ẙẑ 5 53 7 00 0 Bentwisch ᎪܥᎪ 7 07 ܥ 7 35 ܥᎪܥᎪ Ꭺܥ 9 35 ܥᎪܥᎪ Mönchhagen ᎪܥᎪ 7 10 ܥ 7 39 ܥᎪܥᎪ Ꭺܥ 9 39 ܥᎪܥᎪ 15 Rövershagen ẞẖ ܙ 5 05 ܥ 6 04 7 13 ܥ 7 42 ܥ 8 27 ܥ 8 27 9 10 ܥ 9 42 ܥᎪܥᎪ 15 Rövershagen 5 05 ܥ 6 04 7 14 ܥ 8 28 ܥ 8 28 9 11 ܥᎪܥᎪ 20 Gelbensande 5 10 ܥ 6 09 7 18 ܥ 8 35 ܥ 8 35 9 15 ܥᎪܥᎪ 05 10ܥᎪܥ 22 9 48 8 ܥ 48 8 ܥ 24 7 15 6 ܥ 17 5 ܙ Ribnitz-Damgarten West ݘ 29 29 Ribnitz-Damgarten West 5 17 ܥ 6 16 7 25 ܥ 8 49 ܥ 8 49 9 22 ܥᎪܥ10 07 Ꭺܥ 02 10 ܥ 26 9 52 8 ܥ 52 8 ܥ 28 7 19 6 ܥ Ribnitz-Damgarten Ost ݚ 5 21 33 39 Altenwillershagen 5 26 ܥ 6 25 7 32 ܥᎪܥᎪ 9 30 ܥᎪܥᎪ 48 Buchenhorst 5 32 ܥ 6 32 7 38 ܥᎪܥᎪ 9 36 ܥᎪܥᎪ 26 10 ܥ 14 10 ܥ 39 9 02 9 ܥ 02 9 ܥ 41 7 37 6 ܥ 36 5 ܙ Velgast ݚ 54 54 Velgast ẞẍ 5 39 ܥ 6 38 7 42 ܥ 9 03 ܥ 9 03 9 40 ܥ 10 16 ܥ 10 28 63 Martensdorf Ꭺ 5 45 ܥ 6 46 7 49 ܥᎪܥᎪ 9 47 ܥᎪܥᎪ 64 Stralsund-Grünhufe Ꭺ 5 51 ܥ 6 51 7 54 ܥ 9 13 ܥ 9 18 9 53 ܥᎪܥᎪ 41 10 ܥ ẚẍ 10 29 57 9 22 9 ܥ 17 9 ܥ 58 7 55 6 ܥ 55 5 ܙ Stralsund Hbf 193,203,205 ẞẍ ݝ 72 72 Stralsund Hbf ᵜ 10160 ẙẑ 4 59 5 59 ܥ 6 59 7 59 ܥᎪܥᎪ 8 59 ܥ 9 09 9 59 -

Obtaining World Heritage Status and the Impacts of Listing Aa, Bart J.M
University of Groningen Preserving the heritage of humanity? Obtaining world heritage status and the impacts of listing Aa, Bart J.M. van der IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document version below. Document Version Publisher's PDF, also known as Version of record Publication date: 2005 Link to publication in University of Groningen/UMCG research database Citation for published version (APA): Aa, B. J. M. V. D. (2005). Preserving the heritage of humanity? Obtaining world heritage status and the impacts of listing. s.n. Copyright Other than for strictly personal use, it is not permitted to download or to forward/distribute the text or part of it without the consent of the author(s) and/or copyright holder(s), unless the work is under an open content license (like Creative Commons). Take-down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Downloaded from the University of Groningen/UMCG research database (Pure): http://www.rug.nl/research/portal. For technical reasons the number of authors shown on this cover page is limited to 10 maximum. Download date: 23-09-2021 Appendix 4 World heritage site nominations Listed site in May 2004 (year of rejection, year of listing, possible year of extension of the site) Rejected site and not listed until May 2004 (first year of rejection) Afghanistan Península Valdés (1999) Jam, -
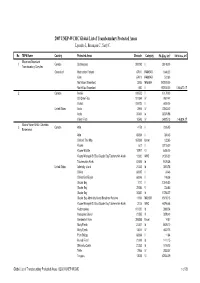
2007 UNEP-WCMC Global List of Transboundary Protected Areas Lysenko I., Besançon C., Savy C
2007 UNEP-WCMC Global List of Transboundary Protected Areas Lysenko I., Besançon C., Savy C. No TBPA Name Country Protected Areas Sitecode Category PA Size, km 2 TBPA Area, km 2 Ellesmere/Greenland 1 Canada Quttinirpaaq 300093 II 38148.00 Transboundary Complex Greenland Hochstetter Forland 67910 RAMSAR 1848.20 Kilen 67911 RAMSAR 512.80 North-East Greenland 2065 MAB-BR 972000.00 North-East Greenland 650 II 972000.00 1,008,470.17 2 Canada Ivvavik 100672 II 10170.00 Old Crow Flats 101594 IV 7697.47 Vuntut 100673 II 4400.00 United States Arctic 2904 IV 72843.42 Arctic 35361 Ia 32374.98 Yukon Flats 10543 IV 34925.13 146,824.27 Alaska-Yukon-British Columbia 3 Canada Atlin 4178 II 2326.95 Borderlands Atlin 65094 II 384.45 Chilkoot Trail Nhp 167269 Unset 122.65 Kluane 612 II 22015.00 Kluane Wildlife 18707 VI 6450.00 Kluane/Wrangell-St Elias/Glacier Bay/Tatshenshini-Alsek 12200 WHC 31595.00 Tatshenshini-Alsek 67406 Ib 9470.26 United States Admiralty Island 21243 Ib 3803.76 Chilkat 68395 II 24.46 Chilkat Bald Eagle 68396 II 198.38 Glacier Bay 1010 II 13045.50 Glacier Bay 22485 V 233.85 Glacier Bay 35382 Ib 10784.27 Glacier Bay-Admiralty Island Biosphere Reserve 11591 MAB-BR 15150.15 Kluane/Wrangell-St Elias/Glacier Bay/Tatshenshini-Alsek 2018 WHC 66796.48 Kootznoowoo 101220 Ib 3868.24 Malaspina Glacier 21555 III 3878.40 Mendenhall River 306286 Unset 14.57 Misty Fiords 21247 Ib 8675.10 Misty Fjords 13041 IV 4622.75 Point Bridge 68394 II 11.64 Russell Fiord 21249 Ib 1411.15 Stikine-LeConte 21252 Ib 1816.75 Tetlin 2956 IV 2833.07 Tongass 13038 VI 67404.09 Global List of Transboundary Protected Areas ©2007 UNEP-WCMC 1 of 78 No TBPA Name Country Protected Areas Sitecode Category PA Size, km 2 TBPA Area, km 2 Tracy Arm-Fords Terror 21254 Ib 2643.43 Wrangell-St Elias 1005 II 33820.14 Wrangell-St Elias 35387 Ib 36740.24 Wrangell-St. -

366 Rügen - Wunderschöne Insel
Süderholzer Blatt mit amtlichen Bekanntmachungen der Gemeinde Süderholz Jahrgang 31 Freitag, den 16. Juli 2021 Nummer 366 Rügen - wunderschöne Insel Die Sommer- und Urlaubszeit hat begonnen kleinen Galerien und Keramikwerkstätten und die Ostseeküste in Mecklenburg-Vor- zu besuchen. Wir waren viel mit Fahrrädern pommern lädt nach vielen Monaten wieder unterwegs, das gefiel, natürlich neben dem Gäste ein. Und die kommen zuhauf. Seit Baden, auch unseren Enkelkindern. Mit langer Zeit schon sind die Hotels und Feri- ihnen teilten wir eine Woche lang unseren enhäuser ausgebucht und auf den Camping- kleinen Wohnwagen. Das fanden sowohl die plätzen bekommt man selten spontan noch Großeltern als auch die Enkel gut. Und das einen Platz. Nicht alle Einheimischen freuen Wetter spielte mit. An den zwei ziemlich küh- sich unbedingt darüber, so manchem wird es len Tagen waren unsere Enkel mit Hilfe des auch mal zu eng auf der Insel. Großvaters sehr kreativ - es entstanden Bilder Ende Juni waren auch wir mal wieder für ein aus Strandgut. Das machte so viel Spaß, dass paar Tage auf Rügen und sind wie immer be- man es nur weiterempfehlen kann. geistert. Rügen - eine Insel, wie sie vielfältiger Nicht nur einmal wurde uns während die- nicht sein kann und immer wieder gibt es Neu- ser Tage bewusst, in was für einem schönen es zu entdecken. Flaches und hügeliges Land Landstrich wir zu Hause sind! Statt hunderter wechseln sich ab, weite Felder gibt es, deren Kilometer, die man aus Bayern und Franken Ende man oft nur erahnen kann. Und dann zurücklegen muss, sind wir im Nu an der Küs- die langen und hellen Strände, Kreidefelsen te. -

ESTUDIO DE LA MICOBIOTA ENDOFÍTICA ASOCIADA a LAS GRAMÍNEAS Dactylis Glomerata, Holcus Lanatus, Ammophila Arenaria Y Elymus Farctus
UNIVERSIDAD DE SALAMANCA FACULTAD DE BIOLOGÍA DEPARTAMENTO DE MICROBIOLOGÍA Y GENÉTICA ESTUDIO DE LA MICOBIOTA ENDOFÍTICA ASOCIADA A LAS GRAMÍNEAS Dactylis glomerata, Holcus lanatus, Ammophila arenaria y Elymus farctus Mª Salud Sánchez Márquez 2009 DR. ÍÑIGO ZABALGOGEAZCOA GONZÁLEZ, CIENTÍFICO TITULAR DEL CONSEJO SUPERIOR DE INVESTIGACIONES CIENTÍFICAS (CSIC), EN EL INSTITUTO DE RECURSOS NATURALES Y AGROBIOLOGÍA DE SALAMANCA, CERTIFICA Que la memoria titulada “ESTUDIO DE LA MICOBIOTA ENDOFÍTICA ASOCIADA A LAS GRAMÍNEAS Dactylis glomerata, Holcus lanatus, Ammophila arenaria y Elymus farctus”, presentada por Dña. Mª Salud Sánchez Márquez para optar al grado de Doctora en Ciencias Biológicas por la Universidad de Salamanca, ha sido realizada bajo mi dirección, en el Departamento de Estrés Abiótico del Instituto de Recursos Naturales y Agrobiología de Salamanca del Consejo Superior de Investigaciones Científicas (CSIC). Y para autorizar su presentación y evaluación por el tribunal correspondiente, expide y firma el presente certificado en Salamanca, a 20 de febrero de 2009. Fdo. Dr. Iñigo Zabalgogeazcoa González ÍNDICE Página 1. INTRODUCCIÓN ……………………………………………………………….. 7 1.1. Aspectos históricos de la investigación sobre hongos endofíticos …………... 10 1.2. Epichloë y Neotyphodium, los hongos endofíticos sistémicos de gramíneas .. 12 1.2.1. Ciclos de vida de Epichloë y Neotyphodium ………………………... 13 1.2.2. Efectos beneficiosos de Epichloë y Neotyphodium …………………. 16 1.2.3. Efectos perjudiciales de los endofitos en el ganado …………………. 18 1.3. Hongos endofíticos no Epichloë …………………………………………….. 19 1.3.1. Abundancia y diversidad taxonómica ………………………………... 20 1.3.2. Especificidad de tejidos ………………………………………………. 21 1.3.3. Especificidad por el hospedador ……………………………………… 22 1.3.4. Transmisión …………………………………………………………... 23 1.3.5. Tipos de interacción planta-hongo endofítico ………………………… 24 1.3.6. -

Quantitative Analysis of the Impact of Fishing Ship Traffic Streams on Traffic Streams of Merchant Vessels in Polish Maritime Areas
Scientific Journals Zeszyty Naukowe of the Maritime University of Szczecin Akademii Morskiej w Szczecinie 2018, 53 (125), 93–101 ISSN 1733-8670 (Printed) Received: 24.10.2017 ISSN 2392-0378 (Online) Accepted: 20.02.2018 DOI: 10.17402/270 Published: 16.03.2018 Quantitative analysis of the impact of fishing ship traffic streams on traffic streams of merchant vessels in Polish maritime areas Anna Anczykowska, Paulina Rekowska, Wojciech Ślączka Maritime University of Szczecin, Faculty of Navigation, Maritime Risk Center 1–2 Wały Chrobrego St., 70-500 Szczecin, Poland e-mail: [email protected] corresponding author Key words: fishing vessels, Baltic Sea, traffic streams, merchant vessels, maritime areas, risk Abstract The Baltic Sea is crisscrossed by several dense vessel traffic routes. Growing shipping traffic increases the likelihood of collisions. A quantitative analysis of the impact of fishing vessel traffic streams on streams of merchant vessel traffic aims to identify areas of intense traffic of this type and to assess the potential risks. The identification of intersections of fishing vessel routes and merchant shipping traffic allows us to identify spots of potential collisions. The analysis made use of the IALA IWRAP Mk2 program and AIS data collected from April 1, 2013 to March 31, 2014. Introduction Shipping routes in the South Baltic – state of knowledge The intensity of commercial vessel traffic in the Baltic Sea has been increasing yearly. The observed The Helsinki Commission (HELCOM) conducts growth comprises mainly bulk carriers, container research on the vessel traffic density in the Baltic ships, general cargo vessels and passenger ships. Sea. Traffic streams are recorded via the automatic Since the LNG terminal in Świnoujście was put into identification system (AIS). -

Spreading of Brine in the Puck Bay in View of In-Situ Measurements
E3S Web of Conferences 54, 00029 (2018) https://doi.org/10.1051/e3sconf/20185400029 SWIM 2018 Spreading of brine in the Puck Bay in view of in-situ measurements Małgorzata Robakiewicz Department of Coastal Engineering and Dynamics, Institute of Hydro-Engineering, Polish Academy of Sciences, Gdańsk, Poland. ABSTRACT Since autumn of 2010 in the north-eastern part of Poland underground gas stores are under construction by diluting salt deposits. A by-product of the technology applied is brine, which is discharged into the coastal waters of the Puck Bay (south Baltic Sea). In the pre- investment study a theoretical analysis of the mixing conditions in the near-field and far- field of the proposed installation was conducted. An extensive monitoring programme carried out since 2010 shows a good mixing of brine with the surrounding waters. Excess salinity due to the continuous discharge of brine estimated using data measured in situ is generally lower than permitted, i.e. not exceeding 0.5 psu in the near-field of installation. INTRODUCTION Increasing demands for gas storage capacity encouraged Polish Gas and Oil Company (PGNiG) to make use of salt deposits located in the north-eastern part of Poland, in the area bordering on the Puck Bay (the inner part of the Gulf of Gdańsk, South Baltic Sea), to create underground gas stores. A complex of 10 chambers (total volume of 250x106 m3) was designed for construction in Kosakowo, near Gdynia. Owing to local geological conditions, the chambers are created at a depth of 800-1600 m. The construction site (GSS, Figure 1) is located about 4 km away from the Baltic Sea coast. -

CHENOPODIACEAE 藜科 Li Ke Zhu Gelin (朱格麟 Chu Ge-Ling)1; Sergei L
Flora of China 5: 351-414. 2003. CHENOPODIACEAE 藜科 li ke Zhu Gelin (朱格麟 Chu Ge-ling)1; Sergei L. Mosyakin2, Steven E. Clemants3 Herbs annual, subshrubs, or shrubs, rarely perennial herbs or small trees. Stems and branches sometimes jointed (articulate); indumentum of vesicular hairs (furfuraceous or farinose), ramified (dendroid), stellate, rarely of glandular hairs, or plants glabrous. Leaves alternate or opposite, exstipulate, petiolate or sessile; leaf blade flattened, terete, semiterete, or in some species reduced to scales. Flowers monochlamydeous, bisexual or unisexual (plants monoecious or dioecious, rarely polygamous); bracteate or ebracteate. Bractlets (if present) 1 or 2, lanceolate, navicular, or scale-like. Perianth membranous, herbaceous, or succulent, (1–)3–5- parted; segments imbricate, rarely in 2 series, often enlarged and hardened in fruit, or with winged, acicular, or tuberculate appendages abaxially, seldom unmodified (in tribe Atripliceae female flowers without or with poorly developed perianth borne between 2 specialized bracts or at base of a bract). Stamens shorter than or equaling perianth segments and arranged opposite them; filaments subulate or linear, united at base and usually forming a hypogynous disk, sometimes with interstaminal lobes; anthers dorsifixed, incumbent in bud, 2-locular, extrorse, or dehiscent by lateral, longitudinal slits, obtuse or appendaged at apex. Ovary superior, ovoid or globose, of 2–5 carpels, unilocular; ovule 1, campylotropous; style terminal, usually short, with 2(–5) filiform or subulate stigmas, rarely capitate, papillose, or hairy on one side or throughout. Fruit a utricle, rarely a pyxidium (dehiscent capsule); pericarp membranous, leathery, or fleshy, adnate or appressed to seed. Seed horizontal, vertical, or oblique, compressed globose, lenticular, reniform, or obliquely ovoid; testa crustaceous, leathery, membranous, or succulent; embryo annular, semi-annular, or spiral, with narrow cotyledons; endosperm much reduced or absent; perisperm abundant or absent.