Report of the 2006 Session of the Joint EIFAC/ICES Working Group on Eels
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Elbe Estuary Publishing Authorities
I Integrated M management plan P Elbe estuary Publishing authorities Free and Hanseatic City of Hamburg Ministry of Urban Development and Environment http://www.hamburg.de/bsu The Federal State of Lower Saxony Lower Saxony Federal Institution for Water Management, Coasts and Conservation www.nlwkn.Niedersachsen.de The Federal State of Schleswig-Holstein Ministry of Agriculture, the Environment and Rural Areas http://www.schleswig-holstein.de/UmweltLandwirtschaft/DE/ UmweltLandwirtschaft_node.html Northern Directorate for Waterways and Shipping http://www.wsd-nord.wsv.de/ http://www.portal-tideelbe.de Hamburg Port Authority http://www.hamburg-port-authority.de/ http://www.tideelbe.de February 2012 Proposed quote Elbe estuary working group (2012): integrated management plan for the Elbe estuary http://www.natura2000-unterelbe.de/links-Gesamtplan.php Reference http://www.natura2000-unterelbe.de/links-Gesamtplan.php Reproduction is permitted provided the source is cited. Layout and graphics Kiel Institute for Landscape Ecology www.kifl.de Elbe water dropwort, Oenanthe conioides Integrated management plan Elbe estuary I M Elbe estuary P Brunsbüttel Glückstadt Cuxhaven Freiburg Introduction As a result of this international responsibility, the federal states worked together with the Federal Ad- The Elbe estuary – from Geeshacht, via Hamburg ministration for Waterways and Navigation and the to the mouth at the North Sea – is a lifeline for the Hamburg Port Authority to create a trans-state in- Hamburg metropolitan region, a flourishing cultural -

Tatenberger Deich Ab
Herzlich willkommen! Hamburger Yacht-Club e.V. im ADAC Hafeninformationen Alles Wissenswerte rund um den HYC für Tagesgäste, Saisongäste, Gastlieger und Mitglieder 1 Inhaltsverzeichnis Herzlich willkommen im Hamburger Yacht-Club! Herzlich willkommen! S. 3 Wir sind ein ehrenamtlich organisierter Anfahrt S. 4–5 Yachthafen für den Motorbootsport an der Dove Elbe. Mit ca. 170 Liegeplätzen Der Yachthafen Tatenberg S. 6 sind wir einer der mitgliederstärksten Umliegende Häfen an der Dove Elbe S. 7 Wassersportclubs Hamburgs. Vereinsleben S. 8–9 Wir bieten Liegeplätze für Yachten Übernachtungskosten S. 10 bis zu 5,50 m Breite und 15 m Länge. Selbstverständlich finden Sie an allen Plätzen Strom- und Hafen-Informationen von A-Z S. 10–14 Wasserversorgung vor. Bei uns liegen Sie mit Ihrem Schiff tidenunabhängig Clubrestaurant S. 15 in sehr geschützter Lage. Versorgung in unmittelbarer Nähe S. 16 Ob mit einem kleinen oder großen Boot, bei uns sind Sie herzlich Hilfreiche Adressen S. 17–18 willkommen, sei es nur für einen Tagesaufenthalt, einen vollen Monat oder Revier Dove Elbe S. 19 auch eine ganze Saison. Sie suchen einen Liegeplatz? Wir finden sicherlich einen geeigneten Platz für Sie – sprechen Sie uns gern an! Dove Elbe retten S. 20 Der Hamburger Yacht-Club ist im ADAC Marineführer gelistet und wurde Freizeit-Tipps S. 20–23 vom DMYV zum Stützpunkt erklärt – wiederholt wurden wir mit dem Mit Bus und Bahn Sightseeing in Hamburg S. 24–25 DMYV-Qualitätssiegel-maritim ausgezeichnet. Impressum S. 25 Über 21 Jahre in Folge hat unser gemeinnütziger Yacht-Club sich den Ansprechpartner S. 26–27 jährlichen internationalen Sicherheits- und Umweltaudits wie z.B. -
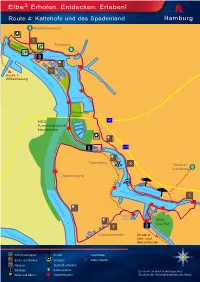
Elbe3 Erholen
Elbe3 Erholen. Entdecken. Erleben! Route 4: Kaltehofe und das Spadenland S Rothenburgsort Entenwerder Elbpark Tiefstack S Billwerder NORDERELBE Bucht Route 1: Wilhelmsburg Holzhafen Billwerder Insel NSG A1 Auenlandschaft Norderelbe A25 Tatenberg Mittlerer Landweg S Spadenland Eichbaumsee Dove-Elbe NORDERELBE Küster- brack NSG Die Reit Gose-Elbe SÜDERELBE Ochsenwerder Route 5: Vier- und Marschlande N Sehenswürdigkeit Strand Hauptroute W O Essen und Trinken Golfplatz Nebenstrecke Museum Yacht-/Bootshafen S S Schleuse S-Bahnstation Die Karte ist nicht maßstabsgetreu. Parks und Gärten Aussichtspunkt Sie dient der Veranschaulichung der Route. Elbe³ Erholen. Entdecken. Erleben! Route 4: Kaltehofe und das Spadenland Mit dem Fahrrad entlang der Norderelbe und Dove-Elbe Wegstrecke: Fahrradrundweg mit leichtem und ebenem Streckenverlauf Start: S-Bahnstation Rothenburgsort Ziel: S-Bahnstation Rothenburgsort Gesamtlänge der Strecke: ca. 29,7 km Ca. Km Teilstrecke Beschreibung der Route 0 km 0,7 km S-Bahnstation Rothenburgsort: links abbiegen auf den Billhorner Deich Besichtigung: Wasserturm und Wassermuseum der Hamburger Wasserwerke GmbH 0,7 km 0,8 km Rechts abbiegen in die Straße Billwerder Neuer Deich 1,5 km 0,1 km Links abbiegen über eine Brücke in den Entenwerder Elbpark 1,6 km 0,9 km Fahrt durch den Elbpark und auf die Straße Entenwerder Stieg 2,5 km 0,1 km Rechts abbiegen in den Ausschläger Elbdeich Betrachtung: Traunsvilla am Traunspark 2,6 km 0,4 km Rechts abbiegen über das Sperrwerk Billwerder Bucht auf den Kaltehofer Hauptdeich Betrachtung: -

Spadenländer Spitze’
‘Spadenländer Spitze’ Measure analysis 09 in the framework of the Interreg IVB project TIDE J. Knüppel 1 1 Hamburg Port Authority (HPA) December 2012 Disclaimer The authors are solely responsible for the content of this report. Material included herein does not represent the opinion of the European Community, and the European Community is not responsible for any use that might be made of it. Johanna Knüppel Hamburg Port Authority (HPA) Citation: Knüppel, J. (2012): ‘Spadenländer Spitze’ (Elbe estuary). Measure analysis in the framework of the Interreg IVB project TIDE. Measure 09. 11 pages. Hamburg. Table of Contents List of tables ............................................................................................................................................. 4 List of figures ............................................................................................................................................ 4 Part 1: Measure description ..................................................................................................................... 5 1.1 Introduction ......................................................................................................................... 5 1.2 Objectives ............................................................................................................................ 5 1.3 Background and side conditions .......................................................................................... 5 1.4 Measure .............................................................................................................................. -

The Barrow+Green-And-Silver 2017 M/Y «Henrik Ibsen» on Irish Rivers and Canals: Part 4 – the Royal Canal + the Shannon
The Barrow+Green-and-Silver 2017 m/y «Henrik Ibsen» on Irish Rivers and Canals: Part 4 – The Royal Canal + the Shannon Exact time for departure from Dublin towards the Royal depends on opening hours for Newcomen Bridge. Newly indicated departure to Spencer Dock is said to be XX. May the lifting of the railway bridge the XX.of May, see detailed plan. Anyway, for "Day XX": Breakfast in GC Dock, crew: local/unclear. After the go-ahead from WI (Waterways Ireland) we leave GC Dock via sea locks, cross the river Liffey and enter the Spencer Dock by sea locks, and under North Wall lifting bridges Spencer Dock (irish: Duga Spencer). The Dock area was originally part of the end of the Royal Canal, which remains connected to the River Liffey here. Spencer Dock includes part of North Wall between Guild Street, Upper Sheriff Street, New Wapping Street, North Wall Quay and Docklands railway station. In agreement with WI we pass by one North Wall Quay lifting bridge, Mayor Street Upper bridge, Sheriff Street Bridge (critical!), several high bridges, before we get to Newcomen railway bridge, popularly called “Effin” Bridge. This is a railway bridge which is very low and normally only open to boats once a month, so this must be booked early. We have ordered the XX.mai. We pass Croke Park Stadium which seats 80,000 spectators and the grim walls of Mountjoy Prison. The canal passes Glasnevin Cemetery at gate 6. - Glasnevin Cemetery is a hidden gem and well worth a visit. It serves today as Ireland's national-cemetery. -

The Book of Irish Poetry
PstiHm liiiill 111 THE BOOK OF IRISH POETRY Drawn 6y] iceo. Morroxv Raftery, the Blind Poet of Connaught Every • Irishman's • Library General Editors: Ai^FRED PercEvai, Graves, m.a. William Magennis, m.a. Douglas Hyde, ll.d. THE BOOK OF IRISH POETRY ior..;<j j"»o.iaii'y i '^ EDITED WITH AN INTRODUCTION BY ALFRED PERCEVAL GRAVES, M.A. T. FISHER UNWIN LONDON: ADELPHI TERRACE LEIPSIC: INSELSTRASSE 20 Printed by Thk Educational Company OF iRKirAND Limited AT The Tai^bot Press Dubinin ; 2?eliicatt0n . To . Douglas Hyde, ll.d., o.utt. Pr*»ident of the Gaelie Leaaue Because, alumni of one Irish College^ And sons of fathers of the self-same Church, Striving to swell the sum of Irish knowledge. Dear Creeveen Eevinn, we unite our search And each of us an Irish Bardic brother In ''Songs of Connachf and "The ' Gael ' has found, This Poem-Book is yours—for to no other By such a kindly friendship am I bound. A. P. G. Of«^o<jy.^ INTRODUCTION. Of anthologies of Irish verse there have been many. Miss Charlotte Brooke's " Irish Poetry," a volume of translations of her own from the Irish, led the way in the year 1789, and was followed by Hardiman's " Irish Minstrelsy," in 183 1 , with metrical translations by Thomas Furlong, Henry Grattan Curran, and John D 'Alton. Both these volumes contained the Irish originals, as well as the translations from them, and both volumes were extremely valuable for their preservation of those originals, but suffered from the over ornate, and, indeed, often extremely artificial English verse into which they were translated. -

Oenanthe Conioides ) Im Bereich Der Freien Und Hansestadt Hamburg
Anlage 2 Freie und Hansestadt Hamburg Behörde für Umwelt, Klima, Energie und Agrarwirtschaft (BUKEA) Hamburg Port Authority (HPA) 03.12.2020 Verbesserungsmaßnahmen für den Schierlings-Wasserfenchel (Oenanthe conioides ) im Bereich der Freien und Hansestadt Hamburg 5. Bericht, Dezember 2020 1. Übersicht über die Verbesserungsmaßnahmen Beim Schierlings-Wasserfenchel (Oenanthe conioides ) handelt es sich um eine priori- täre Art nach FFH-Richtlinie (Anhang I) sowie eine endemische Art, die nur an der Unterelbe vorkommt, da hier aufgrund der Tide im Süßwassermilieu entlang der Fluss- ufer mit ihren Süßwasserwatten geeignete Standortbedingungen vorhanden sind. Um seinen Bestand langfristig zu entwickeln, wurden Maßnahmen geplant und umge- setzt, die entlang des limnischen Abschnitts der Unterelbe zusätzliche Standorte schaffen. Mit Schreiben vom 5.7.2011 hat die Freie und Hansestadt Hamburg die Eu- ropäische Kommission darüber informiert, dass zu diesem Zweck folgende Maßnah- men geplant sind (vgl. Abb. 1-1): • Schaffung eines zweiseitig geöffneten Priels an der Spadenländer Spitze • Entwicklung eines Auenwald-Röhricht-Komplexes mit Schierlings-Wasserfenchel im Bereich Overhaken • Wiederherstellung des Schierlings-Wasserfenchel-Vorkommens im Alten Moorbur- ger Hafen Abb. 1-1: Lage der Verbesserungsmaßnahmen für den Schierlings-Wasserfenchel Über den Stand der Maßnahmen wurde in den vorherigen vier Berichten (2012, 2014, 2016 und 2018) ausführlich informiert. Diese sind unter https://www.kuestendaten.de/Tideelbe/DE/Projekte/FRA20XX/Kompensationsmass- -

TRADITIONAL HERITAGE BOAT SURVEY Grand Canal, Royal Canal, Barrow Navigation
TRADITIONAL HERITAGE BOAT SURVEY Grand Canal, Royal Canal, Barrow Navigation An Initiative of the Waterways Ireland Heritage Plan 2016-2020 In partnership with: Carried out by: Meitheal Mara Crosses Green House Cork [email protected] www.meithealmara.ie 0 Acknowledgements The authors would like to thank the following for their assistance and support in the preparation of this report. Gary Mac Mahon AK Ilen Company Doris McLaughlin Carrick Craft John Beirne, Emerald Star Frank Costelloe Emerald Star Hendrick Boland European Maritime Heritage Boat owners Grand Canal, Royal Canal and Barrow Navigation Liam Hegarty Hegarty’s Boatyard, Oldcourt, Co. Cork Tom Bayly Heritage Boat Association Gerry Burke Heritage Boat Association Cathy Dwane Heritage Boat Association Brian Goggin Heritage Boat Association Paul Martin Heritage Boat Association Conor Nolan Heritage Boat Association Colin Becker Inland Waterways Association of Ireland Reidar Solgvik KonTiki Museum, Oslo Kieran Breen Lough Neagh Heritage Boating Association Rob Robinson St Mullins Mary Swain Shannon Harbour Richard Swain Shannon harbour Paul Kemp Shannon Harbour Justin Connelly Shannon Harbour Frank Durkin Offaly Rowing Club Hugh Hickey Richmond Harbour Cathy Hickey Richmond Harbour Martyn Heighten National Historic Ships, UK Karl Brady National Monuments Service Capt George Hogg National Small Boat Register, UK Hedda Lombardo Norwegian Ship Preservation Society Tim McGuinness Old Gaffers Association Ray Sudcliff The Maritime Trust Criostóir Mac Cárthaigh Traditional Boats of Ireland Hal Sisk Traditional Boats of Ireland The Staff and Board Meitheal Mara The Staff Údarás na Gaelteachta The Staff Waterways Ireland Tina Neylon Editing Pictorial Acknowledgements Photographs were provided by Darina Tully for the survey. The illustrations were commissioned from artist Dónal MacPólin. -

Rapid Risk Assessment HPAIV H5 in Germany, Date 25.03.2021
Rapid Risk Assessment HPAIV H5 in Germany In Germany, around 1,000 HPAIV H5 cases in wild birds and 133 outbreaks in poultry and captive birds have been detected since 30. October 2020. In addition, many European countries continue to report outbreaks of HPAIV subtype H5 in captive birds and wild birds. The risk of spread in waterfowl populations and disease introduction in poultry farms and bird populations (e.g. zoological insti- tutions) is considered high. Currently, there is a high risk of introduction of HPAIV subtype H5 through virus spread between poultry farms (secondary outbreaks). Utmost caution is indicated in (mobile) trade with live poultry. Biosecurity measures in poultry holdings should be reviewed and optimized where possible. Rapid Risk Assessment | FLI | Date 25.03.2021 Rapid Risk Assessment for the introduction and further spread of HPAIV H5 in Germany Background The risk assessment from 22.02.2021 concerned HPAIV H5 events in Germany and Europe. Updated parcels of information are highlighted in yellow and include data up until 23.03.2021. Situation in Germany Since 30 October 2020, HPAIV H5-infected wild birds have been identified predominantly in dead birds on almost a daily basis and subsequently reported via the German Animal Disease Notification System (Tierseuchennachrichtensystem, TSN; Fig. 1). Fourteen federal states are affected thus far (here in chron- ological order of occurrence of cases): Schleswig-Holstein, Hamburg, Mecklenburg-Western Pomerania, Lower Saxony, Brandenburg, Bremen, Saxony, North Rhine-Westphalia, Bavaria, Berlin, Hesse, Baden-Würt- temberg, Thuringia and Rhineland-Palatinate (Fig. 2). The data indicate a supra-regional event that has spread geographically from northern Germany in a southerly direction (Fig. -

Progress at Dunrovin Message by Dave Mccabe Ithe Current Tenants, Well It’S the Time of Year, When We Look the Harringtons Have at the Summer and Reminisce
NEWSLETTER ATHLONE BRANCH INLAND WATERWAYS ASSOCIATION OF IRELAND Autumn 2011 www.athlone.iwai.ie ~ Secretary Michael McDonnell 086 2621136 Chairmans Progress at Dunrovin Message by Dave McCabe IThe current tenants, Well it’s the time of year, when we look the Harringtons have at the summer and reminisce. While the recently left. They summer weather remained dull for most are the last tenants of the time, the various events did manage before the start of to avoid the worst of it. (Perhaps, with the the construction of unfortunate exception of the Richmond the new building. Harbour Visit). The 51st Shannon Rally To protect the was an exceptionally successful event and building from managed to avoid any poor weather. Many unwanted attention and possibly outright vandalism, the structure thanks to the organising committee, its was recently boarded up and made secure. The IWAI is proceeding chairman, Martin Donnelly, Commodore with the development plan, site percolation tests have begun and Tom Meegan and Vice Commodores, the environmental report has been drawn up. It is expected that the Blaithin Fitzgerald and Vivian Clabby. association will formally apply for planning before the end of 2011. I had the opportunity to visit most parts of the Shannon and some of the Royal Athlone Branch IWAI Cruise in this year. The Shannon, especially the harbours in Derg have really benefitted from attention by WI, with new jetties and Company to Cloondara 16th/17th facilities everywhere. Killaloe is a jewel in the crown and worth a visit. July 2011 Following an informal get- We have several forthcoming events so together at Coosan Point jetty please try and attend wherever possible. -

Gewässerökologische Studie Der Elbe (1984)
Arbeitsgemeinschaft für die Reinhaltung der Elbe Schleswig- Holstein Cuxhaven Hamburg Schnackenburg Niedersachsen Gewässerökologische Studie der Elbe von Schnackenburg bis zur See 1984 A R B E I T S G E M E I N S C H A F T F Ü R D I E R E I N H A L T U N G D E R E L B E G E W Ä S S E R Ö K O L O G I S C H E S T U D I E D E R E L B E H y d r o g r a p h i e d e r E l b e - H i s t o r i s c h e E n t w i c k l u n g A u s b a u m a ß n a h m e n u n d d e r e n A u s w i r k u n g e n G e w ä s s e r ö k o l o g i s c h e B e d e u t u n g d e r u n t e r s c h i e d l i c h e n B i o t o p e l e m e n t e d e r E l b e V o r s c h l ä g e z u r E r h a l t u n g u n d V e r b e s s e r u n g d e s a q u a t i s c h e n Ö k o s y s t e m s d e r E l b e ARGE ELBE: Freie und Hansestadt Hamburg Behörde für Bezirksangelegenheiten, Naturschutz und Umweltgestaltung Steindamm 22 2000 H A M B U R G 1 Der Niedersächsische Minister für Ernährung, Landwirtschaft und Forsten Calenbergerstr. -

Effects of the Reconnection of the Dove Elbe to the Tidal Elbe and Local Stakeholders
Effects of the reconnection of the Dove Elbe to the tidal Elbe and local stakeholders Executive summary and lessons learned from a feasibility study Kirsten Wolfstein Victoria Ortiz Hamburg Port Authority Bundesanstalt für Wasserbau Neuer Wandrahm 4 Wedeler Landstraße 157 200547 Hamburg/Germany 22559 Hamburg/Germany February 2021, Hamburg Page 1 of 16 ''This study was supported as part of the IMMERSE project- Implementing Measures for Sustainable Estuaries, an Interreg project supported by the North Sea Programme of the European Regional Development Fund of the European Union Involved consultants: Russeer Weg 54 Tornberg 22 An der Dänischburg 10 24111 Kiel 22337 Hamburg 23569 Lübeck Tel.: 0431/698845 Tel.: 040/41303866 Tel.: 0451/5929800 Fax: 0431/698533 Fax: 040/41303867 Fax: 0451/5929829 www.bbs-umwelt.de www.planung- www.geo-technik.com moderation.eu Tender by: Hamburg Port Authority AoR Neuer Wandrahm 4 20457 Hamburg Page 2 of 16 Content 1. Introduction .................................................................................................................................................. 4 2. Approach ....................................................................................................................................................... 5 3. Results ........................................................................................................................................................... 9 Hydromorphology ............................................................................................................................................