Grins at the Ends of the Earth
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sequencing As a Way of Work
Edinburgh Research Explorer A new insight into Sanger’s development of sequencing Citation for published version: Garcia-Sancho, M 2010, 'A new insight into Sanger’s development of sequencing: From proteins to DNA, 1943-77', Journal of the History of Biology, vol. 43, no. 2, pp. 265-323. https://doi.org/10.1007/s10739-009- 9184-1 Digital Object Identifier (DOI): 10.1007/s10739-009-9184-1 Link: Link to publication record in Edinburgh Research Explorer Document Version: Peer reviewed version Published In: Journal of the History of Biology Publisher Rights Statement: © Garcia-Sancho, M. (2010). A new insight into Sanger’s development of sequencing: From proteins to DNA, 1943-77. Journal of the History of Biology, 43(2), 265-323. 10.1007/s10739-009-9184-1 General rights Copyright for the publications made accessible via the Edinburgh Research Explorer is retained by the author(s) and / or other copyright owners and it is a condition of accessing these publications that users recognise and abide by the legal requirements associated with these rights. Take down policy The University of Edinburgh has made every reasonable effort to ensure that Edinburgh Research Explorer content complies with UK legislation. If you believe that the public display of this file breaches copyright please contact [email protected] providing details, and we will remove access to the work immediately and investigate your claim. Download date: 28. Sep. 2021 THIS IS AN ADVANCED DRAFT OF A PUBLISHED PAPER. REFERENCES AND QUOTATIONS SHOULD ALWAYS BE MADE TO THE PUBLISHED VERION, WHICH CAN BE FOUND AT: García-Sancho M. -

Anfinsen Experiments
Lecture Notes - 2 7.24/7.88J/5.48J The Protein Folding Problem Handouts: An Anfinsen paper Reading List Anfinsen Experiments The Problem of the title refers to how the amino acid sequence of a polypeptide chain determines the folded three-dimensional organization of the chain: • Does the sequence determine the structure?? • Protein Denaturation • Emergence of the Problem • Ribonuclease A • Refolding of ribonuclease in vitro. A. Protein Denaturation/Inactivation One of the features that identified proteins as distinctive polymers was 1) Unusual phase transition in semi purified proteins When exposed to relatively gentle conditions outside the range of physiological • heat • pH • salt • organic solvents, e.g. alcohols Lets consider the familiar transition I mentioned last week; • Heat denaturation; cooking the white of an egg • Acid denaturation; Milk turning sour Macroscopic changes in bulk solution; scatters visible light, increase in viscosity - white of egg: >>heat; opaque, hard; cool down; no change: Coagulation, Aggregation, precipitation, denaturation: One of the components is egg white lysozyme: activity assay; hydrolysis of bacterial cell walls: Activity remaining vs. temperature on same axes This transition: is Denaturation. These transitions were in general found to be irreversible: activity was not recovered upon cooling: Historically the study of the folding and unfolding of proteins emerged from trying to understand this unusual change of state or phase transitions. B. Emergence of the Protein Folding Problem In the period 1958-1960, the first structure of a protein molecule - myoglobin, the oxygen binding protein of muscle - was solved by John Kendrew in 1958, followed soon after by the related tetrameric red blood cell oxygen binding protein, hemoglobin, solved by Max Perutz, both of the British Medical Research Council Labs in Cambridge, U.K. -

Peptide Chemistry up to Its Present State
Appendix In this Appendix biographical sketches are compiled of many scientists who have made notable contributions to the development of peptide chemistry up to its present state. We have tried to consider names mainly connected with important events during the earlier periods of peptide history, but could not include all authors mentioned in the text of this book. This is particularly true for the more recent decades when the number of peptide chemists and biologists increased to such an extent that their enumeration would have gone beyond the scope of this Appendix. 250 Appendix Plate 8. Emil Abderhalden (1877-1950), Photo Plate 9. S. Akabori Leopoldina, Halle J Plate 10. Ernst Bayer Plate 11. Karel Blaha (1926-1988) Appendix 251 Plate 12. Max Brenner Plate 13. Hans Brockmann (1903-1988) Plate 14. Victor Bruckner (1900- 1980) Plate 15. Pehr V. Edman (1916- 1977) 252 Appendix Plate 16. Lyman C. Craig (1906-1974) Plate 17. Vittorio Erspamer Plate 18. Joseph S. Fruton, Biochemist and Historian Appendix 253 Plate 19. Rolf Geiger (1923-1988) Plate 20. Wolfgang Konig Plate 21. Dorothy Hodgkins Plate. 22. Franz Hofmeister (1850-1922), (Fischer, biograph. Lexikon) 254 Appendix Plate 23. The picture shows the late Professor 1.E. Jorpes (r.j and Professor V. Mutt during their favorite pastime in the archipelago on the Baltic near Stockholm Plate 24. Ephraim Katchalski (Katzir) Plate 25. Abraham Patchornik Appendix 255 Plate 26. P.G. Katsoyannis Plate 27. George W. Kenner (1922-1978) Plate 28. Edger Lederer (1908- 1988) Plate 29. Hennann Leuchs (1879-1945) 256 Appendix Plate 30. Choh Hao Li (1913-1987) Plate 31. -

The Early Years-Across the Bench from Bruce (1963-1966)
The Early Years—Across the Bench From Bruce (1963–1966) The Early Years—Across the Bench From Bruce (1963–1966) Garland R. Marshall1,2 1Department of Biochemistry and Molecular Biophysics, Center for Computational Biology, Washington University, St. Louis, MO 63110 2Department of Biomedical Engineering, Center for Computational Biology, Washington University, St. Louis, MO 63110 Received 14 July 2007; revised 20 September 2007; accepted 5 October 2007 Published online 16 October 2007 in Wiley InterScience (www.interscience.wiley.com). DOI 10.1002/bip.20870 a Nobel Laureate, Chairman of the Department of Biology at ABSTRACT: Caltech and a member of the National Academy of Science, and was still willing to recommend me for graduate studies This personal reflection on the author’s experience as at Rockefeller. Bruce Merrifield’s first graduate student has been I was convinced at the time that I was chosen to study adapted from a talk given at the Merrifield Memorial neurophysiology, having failed miserably to isolate the acetyl- Symposium at the Rockefeller University on November choline receptor from denervated rabbit muscle as an under- graduate at Caltech. The outstanding neurophysiologists at 13, 2006. # 2007 Wiley Periodicals, Inc. Biopolymers Rockefeller including H. Keffer Hartline, Nobel Laureate, (Pept Sci) 90: 190–199, 2008. were more interested, however, in the wiring diagrams of the Keywords: solid phase synthesis; Merrifield; DNA synthe- eye of the horseshoe crab2 than in how a small molecule sis; combinatorial chemistry could trigger the action potential. Thus, my first laboratory experience at Rockefeller was with Prof. Henry Kunkel, a This article was originally published online as an accepted prominent immunologist.3 Prof. -

Soccer Schedule 2001
KELLOGG COMMUNITY COLLEGE WOMEN’S BASKETBALL 2021-22 Date Opponent Site MI Time FRI. NOV. 5 BAY DE NOC COMMUNITY COLLEGE BATTLE CREEK, MI 5:30 PM Tues. Nov. 9 Hope College (JV) Holland, MI TBA Weds. Nov. 17 Mott Community College Flint, MI 5:30 pm Weds. Dec. 1 Macomb Community College Warren, MI 5:30 pm Sat. Dec. 11 Lynn Conway Memorial Classic - Schoolcraft College University Center, MI 1:00 pm Sun. Dec. 12 Lynn Conway Memorial Classic - Delta College University Center, MI 4:00 pm WEDS. DEC. 15 MOTT COMMUNITY COLLEGE BATTLE CREEK, MI 5:30 PM Mon. Jan. 3 *Lansing Community College Lansing, MI 7:30 pm WEDS. JAN. 5 *JACKSON COLLEGE BATTLE CREEK, MI 5:30 PM SAT. JAN. 8 *GRAND RAPIDS COMMUNITY COLLEGE BATTLE CREEK, MI 1:00 PM Weds. Jan. 12 *Muskegon Community College Muskegon, MI 5:30 pm SAT. JAN. 15 *KALAMAZOO VALLEY COMMUNITY COLLEGE BATTLE CREEK, MI 1:00 PM WEDS. JAN. 19 *LAKE MICHIGAN COLLEGE BATTLE CREEK, MI 5:30 PM Sat. Jan. 22 *Ancilla College Donaldson, IN 1:00 pm Mon. Jan. 24 *Mid-Michigan Community College Mt. Pleasant, MI 5:30 pm WEDS. JAN. 26 *GLEN OAKS COMMUNITY COLLEGE BATTLE CREEK, MI 5:30 PM SAT. JAN. 29 *LANSING COMMUNITY COLLEGE BATTLE CREEK, MI 1:00 PM Weds. Feb. 2 *Jackson College Jackson, MI 5:30 pm Sat. Feb. 5 *Grand Rapids Community College Grand Rapids, MI 1:00 pm WEDS. FEB. 9 *MUSKEGON COMMUNITY COLLEGE BATTLE CREEK, MI 5:30 PM Sat. Feb. 12 *Kalamazoo Valley Community College Kalamazoo, MI 1:00 pm Weds. -

JUAN MANUEL 2016 NOBEL PEACE PRIZE RECIPIENT Culture Friendship Justice
Friendship Volume 135, № 1 Character Culture JUAN MANUEL SANTOS 2016 NOBEL PEACE PRIZE RECIPIENT Justice LETTER FROM THE PRESIDENT Dear Brothers, It is an honor and a privilege as your president to have the challenges us and, perhaps, makes us question our own opportunity to share my message with you in each edition strongly held beliefs. But it also serves to open our minds of the Quarterly. I generally try to align my comments and our hearts to our fellow neighbor. It has to start with specific items highlighted in each publication. This with a desire to listen, to understand, and to be tolerant time, however, I want to return to the theme “living our of different points of view and a desire to be reasonable, Principles,” which I touched upon in a previous article. As patient and respectful.” you may recall, I attempted to outline and describe how Kelly concludes that it is the diversity of Southwest’s utilization of the Four Founding Principles could help people and “treating others like you would want to be undergraduates make good decisions and build better treated” that has made the organization successful. In a men. It occurred to me that the application of our values similar way, Stephen Covey’s widely read “Seven Habits of to undergraduates only is too limiting. These Principles are Highly Effective People” takes a “values-based” approach to indeed critical for each of us at this particularly turbulent organizational success. time in our society. For DU to be a successful organization, we too, must As I was flying back recently from the Delta Upsilon be able to work effectively with our varied constituents: International Fraternity Board of Directors meeting in undergraduates, parents, alumni, higher education Arizona, I glanced through the February 2017 edition professionals, etc. -

Proquest Dissertations
IMPROVEMENTS TO FLUORESCENT AFFINITY LABELS AND THE RIBONUCLEASE S SYSTEM AND GENE EXPRESSION RESPONSE TO CLINICALLY RELEVANT RIBONUCLEASES by Rex Wayne Watkins A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy (Biochemistry) at the UNIVERSITY OF WISCONSIN-MADISON 2010 UMI Number: 3437078 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. UMT Dissertation Publishing UMI 3437078 Copyright 2010 by ProQuest LLC. All rights reserved. This edition of the work is protected against unauthorized copying under Title 17, United States Code. ProQuest LLC 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 48106-1346 IMPROVEMENTS TO FLUORESCENT AFFINITY LABELS AND THE RIBONUCLEASE S SYSTEM AND GENE EXPRESSION RESPONSE TO CLINICALLY RELEVANT RIBONUCLEASES submitted to the Graduate School of the University of Wisconsin-Madison in partial fulfillment of the requirements for the degree of Doctor of Philosophy By Rex Wayne Watkins Date of final oral examination: ] g August 2010 Month and year degree to be awarded: August 2010 The dissertation is approved by the following members of the Final Oral Committee: Ronald T. Raines, Professor, Biochemistry Aseem Z. Ansari, Professor, Biochemistry Laura L. Kiessling, Professor, Biochemistry Douglas B. Weibel, Professor, Biochemistry Jon S. Thorson, Professor, Pharmaceutical Sciences 1 IMPROVEMENTS TO FLUORESCENT AFFINITY LABELS AND THE RIBONUCLEASE S SYSTEM AND GENE EXPRESSION RESPONSE TO CLINICALLY RELEVANT RIBONUCLEASES Rex Wayne Watkins Under the supervision of Professor Ronald T. -
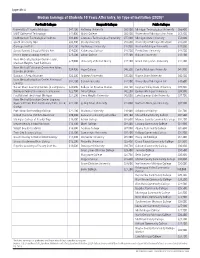
Median Earnings of Students 10 Years After Entry, by Type of Institution (2020)*
Appendix A Median Earnings of Students 10 Years After Entry, by Type of Institution (2020)* For-Profit Colleges Nonprofit Colleges Public Colleges University of Phoenix-Michigan $47,100 Kettering University $80,500 Michigan Technological University $66,400 MIAT College of Technology $41,900 Walsh College $60,400 University of Michigan-Ann Arbor $63,400 Northwestern Technological Institute $33,400 Lawrence Technological University $55,300 Michigan State University $53,600 South University-Novi $33,400 Cleary University $53,600 University of Michigan-Dearborn $48,600 Carnegie Institute $32,100 Northwood University $48,900 Western Michigan University $45,000 Specs Howard School of Media Arts $29,000 Kalamazoo College $48,700 Ferris State University $44,500 Irene's Myomassology Institute $25,600 Albion College $47,400 Oakland University $43,100 Ross Medical Education Center-Taylor, $25,000 University of Detroit Mercy $47,100 Grand Valley State University $42,800 Madison Heights, New Baltimore Ross Medical Education Center-Ann Arbor, $24,900 Hope College $46,200 Central Michigan University $41,900 Davison, Brighton Douglas J Aveda Institute $24,200 Andrews University $45,200 Wayne State University $40,800 Ross Medical Education Center-Kentwood, $24,100 Calvin University $44,800 University of Michigan-Flint $40,600 Lansing Career Quest Learning Centers (4 campuses) $24,000 College for Creative Studies $44,100 Saginaw Valley State University $39,300 Dorsey School of Business (8 campuses) $22,700 Alma College $42,200 Eastern Michigan University -

Kellogg Community College Baseball 2021
KELLOGG COMMUNITY COLLEGE BASEBALL 2021 DATE OPPONENT SITE MI TIME Sun. Feb. 28 Ivy Tech Community College Stevensville, MI 1:00pm Wed. Mar. 3 Ivy Tech Community College (1-9 inning) Fort Wayne, IN 3:00pm Tues. Mar 9 Davenport University (JV) (1-9 inning) Caledonia, MI 3:00pm Fri. Mar. 12 Macomb Community College Detroit PAL 2:00pm Sat. Mar. 13 Macomb Community College Detroit PAL 2:00pm Tues. Mar. 16 Adrian College (JV) Adrian, MI 4:00pm Thurs. Mar. 18 Adrian College (JV) - (1–9 inning) Adrian, MI 4:00pm SUN. MAR. 21 JACKSON COLLEGE BATTLE CREEK, MI 1:00PM FRI. MAR. 26 *LAKE MICHIGAN COLLEGE BATTLE CREEK, MI 2:00PM Sat. Mar. 27 *Lake Michigan College Benton Harbor, MI 1:00pm TUES. MAR. 30 IVY TECH COMMUNITY COLLEGE (1–9 inning) BATTLE CREEK, MI 3:00PM FRI. APR. 2 *GLEN OAKS COMMUNITY COLLEGE BATTLE CREEK, MI 2:00PM Sat. Apr. 3 *Glen Oaks Community College Centreville, MI 1:00pm Tues. Apr. 6 Ivy Tech Community College (1–9 inning) Fort Wayne, IN 3:00pm Fri. Apr. 9 *Kalamazoo Valley Community College Kalamazoo, MI 2:00pm SAT. APR. 10 *KALAMAZOO VALLEY COMMUNITY COLLEGE BATTLE CREEK, MI 1:00PM TUES. APR. 13 MOTT COMMUNITY COLLEGE BATTLE CREEK, MI 2:00PM Fri. Apr. 16 *Grand Rapids Community College Grand Rapids, MI 2:00pm SAT. APR. 17 *GRAND RAPIDS COMMUNITY COLLEGE BATTLE CREEK, MI 1:00PM Tues. Apr. 20 Lansing Community College (1-9 inning) Lansing, MI 3:00pm Fri. Apr. 23 *Muskegon Community College Muskegon, MI 2:00pm SAT. APR. -

The Other Sanger Sequencing; September 2019 1
The Other Sanger Sequencing; September 2019 1 The Other Sanger Sequencing George W. Preston E-mail: [email protected] Earlier this year, a plaque honouring the chromatographers Archer J. P. Martin (1910-2002) and Richard L. M. Synge (1914-1994) appeared in Headingley, Leeds, United Kingdom (Fig. 1A). The plaque, which was unveiled by Leeds Philosophical and Literary Society, can be found on Headingley Lane, near to where the laboratories of the Wool Industries Research Association once stood. It was in these laboratories in the 1940s that Martin and Synge developed partition chromatography – a method for which the pair would receive the 1952 Nobel Prize in Chemistry (Martin, 1952; Synge, 1952). Taking Martin and Synge’s work as a starting point, this article will examine the role of chromatography in efforts to elucidate primary structures of proteins and, latterly, to explore proteomes. Fig. 1. The work of Martin and Synge. (A) The plaque on Headingley Lane; (B) the structure of gramicidin S; (C) the structure of gramicidin S as a series overlapping fragments. PARTITIONING PEPTIDES Partition chromatography is the separation of substances based on how they distribute among different phases. Martin and Synge’s innovation was to support an aqueous phase on a material such as silica or paper, and to pass over this stationary phase an organic mobile phase. They imagined the resulting system as a stack of miniature liquid-liquid extractions, or ‘theoretical plates’, between which the mobile phase (and any analytes dissolved in it) could migrate (Levine, 1963). By enabling the breakdown products of proteins – i.e., amino acids and peptides – to be analysed independently of each other, chromatographic methods greatly accelerated research into protein structure. -
![Elegant Molecules: [Dr. Stanford Moore] Fulvio Bardossi](https://docslib.b-cdn.net/cover/2843/elegant-molecules-dr-stanford-moore-fulvio-bardossi-1792843.webp)
Elegant Molecules: [Dr. Stanford Moore] Fulvio Bardossi
Rockefeller University Digital Commons @ RU Rockefeller University Research Profiles Campus Publications Spring 1982 Elegant Molecules: [Dr. Stanford Moore] Fulvio Bardossi Judith N. Schwartz Follow this and additional works at: http://digitalcommons.rockefeller.edu/research_profiles Part of the Life Sciences Commons Recommended Citation Bardossi, Fulvio and Schwartz, Judith N., "Elegant Molecules: [Dr. Stanford Moore]" (1982). Rockefeller University Research Profiles. Book 13. http://digitalcommons.rockefeller.edu/research_profiles/13 This Article is brought to you for free and open access by the Campus Publications at Digital Commons @ RU. It has been accepted for inclusion in Rockefeller University Research Profiles by an authorized administrator of Digital Commons @ RU. For more information, please contact [email protected]. Carbo I Leu ------Asn--------.....) THE ROCKEFELLER UNIVERSITY I 18 _ C,.---------~~----.-- ....---_...980....101_------~ His (P'o) ~-----------His--- ...../IIB RESEARCH 131 170 s 5 Diagram ofspecial pllDOIi\1CIIES features ofbovine 206 N J.L pancreatic deoxyribonuclease. ......------------Thr SPRING 1982 257 Elegant Molecules Asked how he happened to become a biochemist, Professor FROM THE GREEKPROTEIOS: Stanford Moore attributes the choice to gifted teachers of PRIMARY science and a vocational counselor who advised him that there was no future in aeronautical engineering. "But," he adds Proteins comprise the largest part of the solid mattel ofliving with a smile, ''I've never regretted the choice. I can imagine cells. Some proteins, such as the hemoglobin that carries oxy no life more fascinating or more rewarding than one spent gen in red blood cells, are involved in transport and stOrage. exploring the elegant and complicated architecture of organic Many hormones, like insulin, are proteins; they are chemical molecules." messengers that coordinate body activities. -

Federation Member Society Nobel Laureates
FEDERATION MEMBER SOCIETY NOBEL LAUREATES For achievements in Chemistry, Physiology/Medicine, and PHysics. Award Winners announced annually in October. Awards presented on December 10th, the anniversary of Nobel’s death. (-H represents Honorary member, -R represents Retired member) # YEAR AWARD NAME AND SOCIETY DOB DECEASED 1 1904 PM Ivan Petrovich Pavlov (APS-H) 09/14/1849 02/27/1936 for work on the physiology of digestion, through which knowledge on vital aspects of the subject has been transformed and enlarged. 2 1912 PM Alexis Carrel (APS/ASIP) 06/28/1873 01/05/1944 for work on vascular suture and the transplantation of blood vessels and organs 3 1919 PM Jules Bordet (AAI-H) 06/13/1870 04/06/1961 for discoveries relating to immunity 4 1920 PM August Krogh (APS-H) 11/15/1874 09/13/1949 (Schack August Steenberger Krogh) for discovery of the capillary motor regulating mechanism 5 1922 PM A. V. Hill (APS-H) 09/26/1886 06/03/1977 Sir Archibald Vivial Hill for discovery relating to the production of heat in the muscle 6 1922 PM Otto Meyerhof (ASBMB) 04/12/1884 10/07/1951 (Otto Fritz Meyerhof) for discovery of the fixed relationship between the consumption of oxygen and the metabolism of lactic acid in the muscle 7 1923 PM Frederick Grant Banting (ASPET) 11/14/1891 02/21/1941 for the discovery of insulin 8 1923 PM John J.R. Macleod (APS) 09/08/1876 03/16/1935 (John James Richard Macleod) for the discovery of insulin 9 1926 C Theodor Svedberg (ASBMB-H) 08/30/1884 02/26/1971 for work on disperse systems 10 1930 PM Karl Landsteiner (ASIP/AAI) 06/14/1868 06/26/1943 for discovery of human blood groups 11 1931 PM Otto Heinrich Warburg (ASBMB-H) 10/08/1883 08/03/1970 for discovery of the nature and mode of action of the respiratory enzyme 12 1932 PM Lord Edgar D.