Bycatch Weight, Composition and Preliminary Estimates of the Impact
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lobsters-Identification, World Distribution, and U.S. Trade
Lobsters-Identification, World Distribution, and U.S. Trade AUSTIN B. WILLIAMS Introduction tons to pounds to conform with US. tinents and islands, shoal platforms, and fishery statistics). This total includes certain seamounts (Fig. 1 and 2). More Lobsters are valued throughout the clawed lobsters, spiny and flat lobsters, over, the world distribution of these world as prime seafood items wherever and squat lobsters or langostinos (Tables animals can also be divided rougWy into they are caught, sold, or consumed. 1 and 2). temperate, subtropical, and tropical Basically, three kinds are marketed for Fisheries for these animals are de temperature zones. From such partition food, the clawed lobsters (superfamily cidedly concentrated in certain areas of ing, the following facts regarding lob Nephropoidea), the squat lobsters the world because of species distribu ster fisheries emerge. (family Galatheidae), and the spiny or tion, and this can be recognized by Clawed lobster fisheries (superfamily nonclawed lobsters (superfamily noting regional and species catches. The Nephropoidea) are concentrated in the Palinuroidea) . Food and Agriculture Organization of temperate North Atlantic region, al The US. market in clawed lobsters is the United Nations (FAO) has divided though there is minor fishing for them dominated by whole living American the world into 27 major fishing areas for in cooler waters at the edge of the con lobsters, Homarus americanus, caught the purpose of reporting fishery statis tinental platform in the Gul f of Mexico, off the northeastern United States and tics. Nineteen of these are marine fish Caribbean Sea (Roe, 1966), western southeastern Canada, but certain ing areas, but lobster distribution is South Atlantic along the coast of Brazil, smaller species of clawed lobsters from restricted to only 14 of them, i.e. -
Lobsters LOBSTERS§
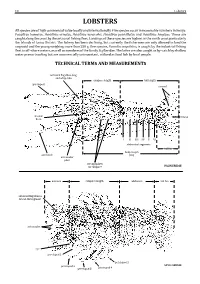
18 Lobsters LOBSTERS§ All species are of high commercial value locally and internationally. Five species occur in reasonable numbers in Kenya: Panulirus homarus, Panulirus ornatus, Panulirus versicolor, Panulirus penicillatus and Panulirus longipes. These are caughtungravid along and the the coast young by weighing the artisanal more fishing than 250 fleet. g. Landings One species, of these Puerulus species angulatus are highest in the north coast particularly the Islands of Lamu District. The fishery has been declining,Scyllaridae. but currently The latter the fishermen are also caught are only as by–catch allowed toby landshallow the , is caught by the industrial fishing fleet in off–shore waters, as well as members of the family water prawn trawling but areTECHNICAL commercially unimportant, TERMS AND utilized MEASUREMENTS as food fish by local people. and whip–like antennal flagellum long carapace length tail length pereiopod uropod frontal telson horn III III IV VIV abdominal segments tail fan body length antennule (BL) antennular plate strong spines on carapace PALINURIDAE antenna carapace length abdomen tail fan antennal flagellum a broad, flat segment antennules eye pereiopod 1 pereiopod 5 pereiopod 2 SCYLLARIDAE pereiopod 3 pereiopod 4 Guide to Families 19 GUIDE TO FAMILIES NEPHROPIDAE Page 20 True lobsters § To about 15 cm. Marine, mainly deep waters on soft included in the Guide to Species. 1st pair of substrates. Three species of interest to fisheriespereiopods are large 3rd pair of pereiopods with chela PALINURIDAE Page 21 Antennal Spiny lobsters § To about 50 cm. Marine, mostly shallow waters on flagellum coral and sand stone reefs, some species on soft included in the Guide to Species. -

ESTADÍSTICO DE PESCA Índice
ANUARIO ESTADÍSTICO DE PESCA Índice INTRODUCCIÓN 7 CAPÍTULO I PRODUCCIÓN PESQUERA 13 CAPÍTULO II INDUSTRIALIZACIÓN 109 CAPÍTULO III COMERCIALIZACIÓN Y CONSUMO 123 CAPÍTULO IV FACTORES DE PRODUCCIÓN 147 CAPÍTULO V NORMATIVIDAD 177 CAPÍTULO VI ESTADÍSTICAS INTERNACIONALES 201 GLOSARIO 237 ÍNDICE DE CUADROS 243 ANEXO 257 SECRETARÍA DE AGRICULTURA, GANADERÍA, DESARROLLO RURAL, PESCA Y ALIMENTACIÓN Javier Bernardo Usabiaga Arroyo SECRETARIO Jerónimo Ramos Sáenz Pardo COMISIONADO NACIONAL DE ACUACULTURA Y PESCA Juan Carlos Cortés García SUBSECRETARIO DE PLANEACIÓN Víctor Villalobos Arámbula SUBSECRETARIO DE AGRICULTURA Y GANADERÍA Antonio Ruiz García SUBSECRETARIO DE DESARROLLO RURAL Mara Angélica Murillo Correa DIRECTORA GENERAL DE POLÍTICA Y FOMENTO PESQUERO Guillermo Compean Jiménez PRESIDENTE DEL INSTITUTO NACIONAL DE LA PESCA p Introducción Introducción a Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación, tiene como uno de sus propósitos esenciales difundir en forma confiable y oportuna, los L principales indicadores de la actividad pesquera en México, que son importantes para conocer el comportamiento y evolución de la explotación, conservación e industrialización de la flora y fauna acuática del país. La SAGARPA a través del desarrollo y actualización de su infraestructura informática y el rediseño de los sistemas estadísticos, aunado a la automatización en sus procesos, propicia las condiciones necesarias para la generación de información estadística actual y confiable, que permite conocer los fenómenos que comprende la pesca en su conjunto. Para la integración de este documento fue necesaria una cercana vinculación entre las delegaciones federales, las oficinas de la SAGARPA y los órganos centrales de la Secretaría, quienes por medio de procedimiento ya establecido, llevaron a cabo la tarea de recopilar e integrar la información estadística emanada de los diferentes agentes que participan activamente en este sector. -

Goldstein Et Al 2019
Journal of Crustacean Biology Advance Access published 24 August 2019 Journal of Crustacean Biology The Crustacean Society Journal of Crustacean Biology 39(5), 574–581, 2019. doi:10.1093/jcbiol/ruz055 Downloaded from https://academic.oup.com/jcb/article-abstract/39/5/574/5554142/ by University of New England Libraries user on 04 October 2019 Development in culture of larval spotted spiny lobster Panulirus guttatus (Latreille, 1804) (Decapoda: Achelata: Palinuridae) Jason S. Goldstein1, Hirokazu Matsuda2, , Thomas R. Matthews3, Fumihiko Abe4, and Takashi Yamakawa4, 1Wells National Estuarine Research Reserve, Maine Coastal Ecology Center, 342 Laudholm Farm Road, Wells, ME 04090 USA; 2Mie Prefecture Fisheries Research Institute, 3564-3, Hamajima, Shima, Mie 517-0404 Japan; 3Florida Fish and Wildlife Conservation Commission, Fish and Wildlife Research Institute, 2796 Overseas Hwy, Suite 119, Marathon, FL 33050 USA; and 4Department of Aquatic Bioscience, Graduate School of Agricultual and Life Sciences, The University of Tokyo, 1-1-1 Yayoi, Bunkyo, Tokyo 113-8657 Japan HeadA=HeadB=HeadA=HeadB/HeadA Correspondence: J.S. Goldstein: e-mail: [email protected] HeadB=HeadC=HeadB=HeadC/HeadB (Received 15 May 2019; accepted 11 July 2019) HeadC=HeadD=HeadC=HeadD/HeadC Ack_Text=DisHead=Ack_Text=HeadA ABSTRACT NList_lc_rparentheses_roman2=Extract1=NList_lc_rparentheses_roman2=Extract1_0 There is little information on the early life history of the spotted spiny lobster Panulirus guttatus (Latreille, 1804), an obligate reef resident, despite its growing importance as a fishery re- BOR_HeadA=BOR_HeadB=BOR_HeadA=BOR_HeadB/HeadA source in the Caribbean and as a significant predator. We cultured newly-hatched P. guttatus BOR_HeadB=BOR_HeadC=BOR_HeadB=BOR_HeadC/HeadB larvae (phyllosomata) in the laboratory for the first time, and the growth, survival, and mor- BOR_HeadC=BOR_HeadD=BOR_HeadC=BOR_HeadD/HeadC phological descriptions are reported through 324 days after hatch (DAH). -

Taxonomy, Biology and Distribution of Lobsters
Taxonomy, Biology and Distribution of Lobsters 15 Rekha Devi Chakraborty and E.V.Radhakrishnan Crustacean Fisheries Division, Central Marine Fisheries Research Institute, Kochi-682 018 Lobsters are among the most prized of fisheries resources and of significant commercial interest in many countries. Because of their high value and esteemed culinary worth, much attention has been paid to lobsters in biological, fisheries, and systematic literature. They have a great demand in the domestic market as a delicacy and is a foreign exchange earner for the country. Taxonomic status Phylum: Arthropoda Subphylum: Crustacea Class: Malacostraca Subclass: Eumalacostraca Superorder: Eucarida Order: Decapoda Suborder: Macrura Reptantia The suborder Macrura Reptantia consists of three infraorders: Astacidea (Marine lobsters and freshwater crayfishes), Palinuridea (Spiny lobsters and slipper lobsters) and Thalassinidea (mud lobsters). The infraorder Astacidea Summer School on Recent Advances in Marine Biodiversity Conservation and Management 100 Rekha Devi Chakraborty and E.V.Radhakrishnan contains three superfamilies of which only one (the Infraorder Palinuridea, Superfamily Eryonoidea, Family Nephropoidea) is considered here. The remaining two Polychelidae superfamilies (Astacoidea and parastacoidea) contain the 1b. Third pereiopod never with a true chela,in most groups freshwater crayfishes. The superfamily Nephropoidea (40 chelae also absent from first and second pereiopods species) consists almost entirely of commercial or potentially 3a Antennal flagellum reduced to a single broad and flat commercial species. segment, similar to the other antennal segments ..... Infraorder Palinuridea, Superfamily Palinuroidea, The infraorder Palinuridea also contains three superfamilies Family Scyllaridae (Eryonoidea, Glypheoidea and Palinuroidea) all of which are 3b Antennal flagellum long, multi-articulate, flexible, whip- marine. The Eryonoidea are deepwater species of insignificant like, or more rigid commercial interest. -

Historic Naturalis Classica, Viii Historic Naturalis Classica
HISTORIC NATURALIS CLASSICA, VIII HISTORIC NATURALIS CLASSICA EDIDERUNT J. CRAMER ET H.K.SWANN TOMUS vm BIBUOGRAPHY OF THE LARVAE OF DECAPOD CRUSTACEA AND LARVAE OF DECAPOD CRUSTACEA BY ROBERT GURNEY WITH 122 FIGURES IN THE TEXT REPRINTED 1960 BY H. R. ENGELMANN (J. CRAMER) AND WHELDON & WESLEY, LTD. WEINHEIM/BERGSTR. CODICOTE/HERTS. BIBLIOGRAPHY OF THE LARVAE OF DECAPOD CRUSTACEA AND LARVAE OF DECAPOD CRUSTACEA BY ROBERT GURNEY WITH 122 FIGURES IN THE TEXT REPRINTED 1960 BY H. R. ENGELMANN (J. CRAMER) AND WHELDON & WESLEY, LTD. WEINHEIM/BERGSTR. CODICOTE/HERTS. COPYRIGHT 1939 & 1942 BY THfi RAY SOCIETY IN LONDON AUTHORIZED REPRINT COPYRIGHT OF THE SERIES BY J. CRAMER PUBLISHER IN WEINHEIM PRINTED IN GERMANY I9«0 i X\ T • THE RAY SOCIETY INSTITUTED MDCCCXLIV This volume (No. 125 of the Series) is issued to the Svhscribers to the RAY SOCIETY JOT the Year 1937. LONDON MCMXXXIX BIBLIOGKAPHY OF THE LARVAE OF DECAPOD CRUSTACEA BY ROBERT GURNEY, M.A., D.Sc, F.L.S. LONDON PRINTED FOR THE RAT SOCIETY SOLD BT BERNARD QUARITCH, LTD. U, GBAFTOK STBKET, NBW BOND STEBBT, LONDON, "W. 1 1939 PRINTED BY ADLABD AND SON, LIMITED 2 1 BLOOJlSBUBY WAY, LONDON, W.C. I Madt and printed in Great Britain. CONTENTS PAOE PBBFACE . " V BiBUOGRAPHY CLASSIFIED LIST . 64 Macrura Natantia 64 Penaeidea 64 Caridea 70 Macrura Reptantia 84 Nephropsidea 84 Eryonidea 88 Scyllaridea 88 Stenopidea 91 Thalassinidea 92 Anomura ; 95 Galatheidea . 95 Paguridea 97 Hippidea 100 Dromiacea 101 Brachyura 103 Gymnopleura 103 Brachygnatha 103 Oxyrhyncha 113 Oxystomata . 116 INDEX TO GENERA 120 PREFACE IT has been my intention to publish a monograph of Decapod larvae which should contain a bibliography, a part dealing with a number of general questions relating to the post-embryonic development of Decapoda and Euphausiacea, and a series of sections describing the larvae of all the groups, so far as they are known. -

Developmental Changes in the Mouthparts of Juvenile Caribbean Spiny Lobster, Panulirus Argus: Implications for Aquaculture
FAD LIBRARIES Florida Atlantic University HARBOR BRANCH ~ FLORIDA ATLANTIC UNIVERSITY FAU Institutional Repository http://purl.fcla.edu/fau/fauir This paper was submitted by the faculty of FAU’s Harbor Branch Oceanographic Institute. Notice: ©2008 Elsevier B.V. The final published version of this manuscript is available at http://www.sciencedirect.com/science/journal/00448486. This article may be cited as: Cox, S. L., Jeffs, A. G., & Davis, M. (2008). Developmental changes in the mouthparts of juvenile Caribbean spiny lobster, Panulirus argus: Implications for aquaculture. Aquaculture, 283(1‐4), 168‐174. doi:10.1016/j.aquaculture.2008.07.019 Aquaculture 283 (2008) 168–174 Contents lists available at ScienceDirect Aquaculture journal homepage: www.elsevier.com/locate/aqua-online Developmental changes in the mouthparts of juvenile Caribbean spiny lobster, Panulirus argus: Implications for aquaculture Serena L. Cox a,b,⁎, Andrew G. Jeffs c, Megan Davis a a Aquaculture Division, Harbor Branch Oceanographic Institute at Florida Atlantic University, 5600 US 1 North, Fort Pierce, Florida 34946, USA b National Institute of Water and Atmospheric Research, Private Bag 14901, Kilbirnie, Wellington 6241, New Zealand c Leigh Marine Laboratory, University of Auckland, P.O. Box 349, Warkworth 0941, New Zealand article info abstract Article history: Light microscopy and video analysis were used to examine the mouthpart morphology and feeding Received 5 May 2008 behaviour of the Caribbean spiny lobster from puerulus (megalopal stage) (5–8 mm carapace length, CL) to Received in revised form 14 July 2008 adult (85 mm CL). Upon settlement the pueruli did not possess fully functional mouthparts, however, Accepted 14 July 2008 efficient feeding appendages appeared in the first instar juvenile (after the first moult from puerulus). -

The Massive Death of Lobsters Smothered Within Their Thalassinoides Burrows: the Example of the Lower Barremian from Lusitanian Basin (Portugal)
Versão online: http://www.lneg.pt/iedt/unidades/16/paginas/26/30/209 Comunicações Geológicas (2016) 103, Especial I, 143-152 ISSN: 0873-948X; e-ISSN: 1647-581X The massive death of lobsters smothered within their Thalassinoides burrows: the example of the lower Barremian from Lusitanian Basin (Portugal) A morte em massa de lagostas sepultadas no interior das suas galerias do tipo Thalassinoides: o exemplo do Barremiano inferior da Bacia Lusitânica (Portugal) C. Neto de Carvalho 1,* Artigo original © 2014 LNEG – Laboratório Nacional de Geologia e Energia IP Original Article Abstract: Some years ago an exceptional site with the preservation of the evolutiva demonstrada pela ocorrência de Thalassinoides associados a lagostas mecochirid lobster Meyeria rapax (Harbort) within their burrows was glyphoídeos desde o Jurássico Superior ao Aptiano, e à sua progressiva described in the Early Cretaceous of Cabo Espichel sector, Lusitanian Basin, substituição pelos thalassinídeos, com algumas excepções registadas para as Portugal. The large number of articulated specimens preserved at the bottom lagostas queladas, a partir do Cretácico Superior. Esta tendência para a of Thalassinoides burrow systems in, at least, four different beds of the Boca convergência evolutiva no hábito fossorial é comparável com os padrões do Chapim Formation (lower Barremian) suggests that a sudden cyclic event evolutivos de diversificação das lagostas e dos thalassinídeos reconhecidos was responsible for the collective killing below the bottom of a confined recentemente. lagoon, and led to the very rare preservation of both burrows and producers. Palavras-chave: Meyeria rapax ; Thalassinoides suevicus ; morte colectiva; The explanation may be found in the highly heterogranular, coarse-grained convergência comportamental; Barremiano inferior; Bacia Lusitânica clastic filling of the burrow systems which represent influxes of freshwater and large volumes of siliciclastics that may had suddenly drop the salinity 1 Geopark Naturtejo da Meseta Meridional – UNESCO Global Geopark. -

Digging Mechanisms and Substrate Preferences of Shovel Nosed Lobsters, Ibacus Peronii (Decapoda: Scyllaridae)
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Scholarworks@UTRGV Univ. of Texas RioGrande Valley University of Texas Rio Grande Valley ScholarWorks @ UTRGV Biology Faculty Publications and Presentations College of Sciences 1-2006 Digging Mechanisms and Substrate Preferences of Shovel Nosed Lobsters, Ibacus peronii (Decapoda: Scyllaridae) Zen Faulkes The University of Texas Rio Grande Valley, [email protected] Follow this and additional works at: https://scholarworks.utrgv.edu/bio_fac Part of the Biology Commons Recommended Citation Faulkes, Zen, "Digging Mechanisms and Substrate Preferences of Shovel Nosed Lobsters, Ibacus peronii (Decapoda: Scyllaridae)" (2006). Biology Faculty Publications and Presentations. 34. https://scholarworks.utrgv.edu/bio_fac/34 This Article is brought to you for free and open access by the College of Sciences at ScholarWorks @ UTRGV. It has been accepted for inclusion in Biology Faculty Publications and Presentations by an authorized administrator of ScholarWorks @ UTRGV. For more information, please contact [email protected], [email protected]. JOURNAL OF CRUSTACEAN BIOLOGY, 26(1): 69–72, 2006 DIGGING MECHANISMS AND SUBSTRATE PREFERENCES OF SHOVEL NOSED LOBSTERS, IBACUS PERONII (DECAPODA: SCYLLARIDAE) Zen Faulkes Department of Zoology, University of Melbourne, Royal Parade, Parkville, Victoria, 3010, Australia; Present address: Department of Biology, University of Texas – Pan American, 1201 W. University Drive, Edinburg, Texas, 78541, U.S.A. ([email protected]) ABSTRACT Digging is a distinct form of locomotion that poses different mechanical problems than other locomotor modes that are commonly used by crustaceans, e.g., walking, swimming. I examined the mechanisms of digging by shovel nosed lobsters (Ibacus peronii), which spend most of the day underneath sand. -

Late Cretaceous and Paleocene Decapod Crustaceans from James Ross Basin, Antarctic Peninsula Author(S): Rodney M
Paleontological Society Late Cretaceous and Paleocene Decapod Crustaceans from James Ross Basin, Antarctic Peninsula Author(s): Rodney M. Feldmann, Dale M. Tshudy, Michael R. A. Thomson Source: Memoir (The Paleontological Society), Vol. 28, Supplement to Vol. 67, no. 1 of the Journal of Paleontology (Jan., 1993), pp. 1-41 Published by: Paleontological Society Stable URL: http://www.jstor.org/stable/1315582 Accessed: 16/01/2009 20:00 Your use of the JSTOR archive indicates your acceptance of JSTOR's Terms and Conditions of Use, available at http://www.jstor.org/page/info/about/policies/terms.jsp. JSTOR's Terms and Conditions of Use provides, in part, that unless you have obtained prior permission, you may not download an entire issue of a journal or multiple copies of articles, and you may use content in the JSTOR archive only for your personal, non-commercial use. Please contact the publisher regarding any further use of this work. Publisher contact information may be obtained at http://www.jstor.org/action/showPublisher?publisherCode=paleo. Each copy of any part of a JSTOR transmission must contain the same copyright notice that appears on the screen or printed page of such transmission. JSTOR is a not-for-profit organization founded in 1995 to build trusted digital archives for scholarship. We work with the scholarly community to preserve their work and the materials they rely upon, and to build a common research platform that promotes the discovery and use of these resources. For more information about JSTOR, please contact [email protected]. Paleontological Society is collaborating with JSTOR to digitize, preserve and extend access to Memoir (The Paleontological Society). -

ZM 72-10 (Brown) 05-01-2007 10:44 Page 113
ZM 72-10 (Brown) 05-01-2007 10:44 Page 113 The Australian species of the genus Ibacus (Crustacea: Decapoda: Scyllaridae), with the description of a new species and addition of new records D.E. Brown & L.B. Holthuis Brown, D.E. & L.B. Holthuis. The Australian species of the genus Ibacus (Crustacea: Decapoda: Scyl- laridae), with the description of a new species and addition of new records. Zool. Med. Leiden 72 (10), 11.xii.1998: 113-141, figs 1-3, pls 1-10.— ISSN 0024-0672. Diane E. Brown, Australian Museum, 6 College Street, Sydney, 2000 Australia. L.B. Holthuis, Nationaal Natuurhistorisch Museum, Naturalis, P.O. Box 9517, 2300 RA Leiden, The Netherlands. Key words: Decapoda; Scyllaridae; Ibacus; new species; Australia. A new species of scyllarid lobster, Ibacus chacei, from eastern Australia is described and illustrated. The new species can be distinguished from all other known Ibacus species by the shape of the third maxilliped. Ibacus brevipes Bate, 1888 is recorded from Australia for the first time. Seven out of the eight known species of Ibacus are now recorded from Australia. Colour descriptions, and updated dis- tributions for all Australian Ibacus species are included, plus further comments on the type locality of Ibacus peronii Leach, 1815. Colour illustrations and a key to the eight known species of Ibacus are also provided. Introduction The genus Ibacus occurs only in the Indo-West Pacific region (Holthuis 1985, 1991), and until the mid 1970’s, only two species, I. peronii Leach, 1815 and I. alticrena- tus Bate, 1888 were recorded from Australian waters. -

Two Newly Recorded Species of the Lobster Family Scyllaridae (Thenus Indicus and Scyllarides Haanii) from South of Java, Indonesia
HAYATI Journal of Biosciences 23 (2016) 101e105 HOSTED BY Contents lists available at ScienceDirect HAYATI Journal of Biosciences journal homepage: http://www.journals.elsevier.com/ hayati-journal-of-biosciences Original research article Two Newly Recorded Species of the Lobster Family Scyllaridae (Thenus indicus and Scyllarides haanii) From South of Java, Indonesia * Yusli Wardiatno, Agus Alim Hakim, Ali Mashar, Nurlisa Alias Butet, Luky Adrianto Department of Aquatic Resources Management, Faculty of Fisheries and Marine Science, Bogor Agricultural University, Bogor, West Java, Indonesia. article info abstract Article history: Two species of slipper lobster, Thenus indicus Leach, 1815, and Scyllarides haanii De Haan, 1841, are re- Received 5 February 2016 ported for the first time from the coastal waters of South of Java, part of the Indian Ocean. A total of two Received in revised form specimens, one specimen of T. indicus from Palabuhanratu Bay and one specimen of S. haanii from 30 April 2016 Yogyakarta coastal waters, were collected in April and September 2015, respectively. Descriptions and Accepted 9 May 2016 illustrations of the morphological characteristics of the two species and their habitat are presented. Available online 26 May 2016 Copyright © 2016 Institut Pertanian Bogor. Production and hosting by Elsevier B.V. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/). KEYWORDS: Crustacean, Decapoda, first record, Indian Ocean, slipper lobster 1. Introduction only one species, Thenus orientalis. Taxonomic and biodiversity studies on Indonesian lobsters resulted in the collection of two From a fishery point of view, slipper lobsters of the family different species of family Scyllaridae from South of Java.