Goldstein Et Al 2019
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

U , '' Regional (;"Ntre of I:::;~, I Central Marine Fisheries Research Institute T - ~ '\~ ~ ~3L'!" ICAR Marine Fisheries P.O
CMFRI S ammelt S ,4(J(Jt (JU Recent Advances in Se"ed Production and Growout Techniques for Marine Finfish and Shellfish ,1 I Compiled and Edited by Dr. G.Gopakumar, Principal Scientist & Director, Summer School and '. Dr. Boby Ignatius, Scientist (SS) Central Marine Fisheries Research Institute j' U , '' Regional (;"ntre of i:::;~, I Central Marine Fisheries Research Institute t - ~ '\~ ~ ~3l'!" ICAR Marine Fisheries P.O . Tamil Nadu - 623 520 ' ~~ ... ~"'~~ SEED PRODUCTION OF THE SAND LOBSTER THENUS ORIENTALIS (LUND) Joe K. Kizhakudan Research Centre of CMFRI, Chennai 3f With the decline in many commercial fisheries worldwide and an ever increasing demand for seafood protein, there is a growing need for augmenting the production of high-protein, high-value resources like lobsters. Aquaculture remains the ideal measure to augment production and ensure conseNation, and even enhancement. of natural stocks. Aquaculture provides a two-pronged solution towards increasing the fish production through ., farming of hatchery-produced seed of commercially important finfishes and shellfishes , enhancing natural stocks by sea ranching hatchery-produced seed of commercially important finfishes and shellfishes Lobsters are among the most priced seafood delicacies enjoying a special demand in international markets. As against a world average annual productio'n of2.1 lakh tonnes, India's average annual lobster production is about 2000 tonnes. With the distinction of being perhaps, the only seafood resource in India's trade economy, which remains relatively low down the ladder in terms of quantity of production but brings in maximum foreign exchange, lobsters have been the subject of study for more than two decades now. -

Lobsters-Identification, World Distribution, and U.S. Trade
Lobsters-Identification, World Distribution, and U.S. Trade AUSTIN B. WILLIAMS Introduction tons to pounds to conform with US. tinents and islands, shoal platforms, and fishery statistics). This total includes certain seamounts (Fig. 1 and 2). More Lobsters are valued throughout the clawed lobsters, spiny and flat lobsters, over, the world distribution of these world as prime seafood items wherever and squat lobsters or langostinos (Tables animals can also be divided rougWy into they are caught, sold, or consumed. 1 and 2). temperate, subtropical, and tropical Basically, three kinds are marketed for Fisheries for these animals are de temperature zones. From such partition food, the clawed lobsters (superfamily cidedly concentrated in certain areas of ing, the following facts regarding lob Nephropoidea), the squat lobsters the world because of species distribu ster fisheries emerge. (family Galatheidae), and the spiny or tion, and this can be recognized by Clawed lobster fisheries (superfamily nonclawed lobsters (superfamily noting regional and species catches. The Nephropoidea) are concentrated in the Palinuroidea) . Food and Agriculture Organization of temperate North Atlantic region, al The US. market in clawed lobsters is the United Nations (FAO) has divided though there is minor fishing for them dominated by whole living American the world into 27 major fishing areas for in cooler waters at the edge of the con lobsters, Homarus americanus, caught the purpose of reporting fishery statis tinental platform in the Gul f of Mexico, off the northeastern United States and tics. Nineteen of these are marine fish Caribbean Sea (Roe, 1966), western southeastern Canada, but certain ing areas, but lobster distribution is South Atlantic along the coast of Brazil, smaller species of clawed lobsters from restricted to only 14 of them, i.e. -
Lobsters LOBSTERS§
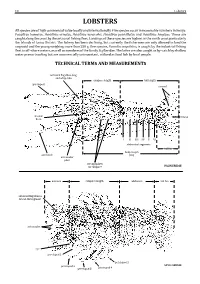
18 Lobsters LOBSTERS§ All species are of high commercial value locally and internationally. Five species occur in reasonable numbers in Kenya: Panulirus homarus, Panulirus ornatus, Panulirus versicolor, Panulirus penicillatus and Panulirus longipes. These are caughtungravid along and the the coast young by weighing the artisanal more fishing than 250 fleet. g. Landings One species, of these Puerulus species angulatus are highest in the north coast particularly the Islands of Lamu District. The fishery has been declining,Scyllaridae. but currently The latter the fishermen are also caught are only as by–catch allowed toby landshallow the , is caught by the industrial fishing fleet in off–shore waters, as well as members of the family water prawn trawling but areTECHNICAL commercially unimportant, TERMS AND utilized MEASUREMENTS as food fish by local people. and whip–like antennal flagellum long carapace length tail length pereiopod uropod frontal telson horn III III IV VIV abdominal segments tail fan body length antennule (BL) antennular plate strong spines on carapace PALINURIDAE antenna carapace length abdomen tail fan antennal flagellum a broad, flat segment antennules eye pereiopod 1 pereiopod 5 pereiopod 2 SCYLLARIDAE pereiopod 3 pereiopod 4 Guide to Families 19 GUIDE TO FAMILIES NEPHROPIDAE Page 20 True lobsters § To about 15 cm. Marine, mainly deep waters on soft included in the Guide to Species. 1st pair of substrates. Three species of interest to fisheriespereiopods are large 3rd pair of pereiopods with chela PALINURIDAE Page 21 Antennal Spiny lobsters § To about 50 cm. Marine, mostly shallow waters on flagellum coral and sand stone reefs, some species on soft included in the Guide to Species. -

Multiple Measures of Thermal Performance of Early Stage Eastern Rock Lobster in a Fast-Warming Ocean Region
Vol. 624: 1–11, 2019 MARINE ECOLOGY PROGRESS SERIES Published August 15 https://doi.org/10.3354/meps13054 Mar Ecol Prog Ser OPENPEN ACCESSCCESS FEATURE ARTICLE Multiple measures of thermal performance of early stage eastern rock lobster in a fast-warming ocean region Samantha Twiname1,*, Quinn P. Fitzgibbon1, Alistair J. Hobday2, Chris G. Carter1, Gretta T. Pecl1 1Institute for Marine and Antarctic Studies, University of Tasmania, Private Bag 49, Hobart, TAS 7001, Australia 2CSIRO Oceans and Atmosphere, Castray Esplanade, Hobart, TAS 7001, Australia ABSTRACT: To date, many studies trying to under- stand species’ climate-driven changes in distribution, or ‘range shifts’, have each focused on a single poten- tial mechanism. While a single performance measure may give some insight, it may not be enough to ac - curately predict outcomes. Here, we used multiple measures of performance to explore potential mech- anisms behind species range shifts. We examined the thermal pattern for multiple measures of performance, including measures of aerobic metabolism and multi- ple as pects of escape speed, using the final larval stage (puerulus) of eastern rock lobster Sagmariasus verreauxi as a model species. We found that aerobic scope and escape speed had different thermal per- Single thermal performance measures for eastern rock lob- formances and optimal temperatures. The optimal ster Sagmariasus verreauxi may not accurately predict whole temperature for aerobic scope was 27.5°C, while the animal performance under future ocean warming. pseudo-optimal temperature for maximum escape Photo: Peter Mathew speed was 23.2°C. This discrepancy in thermal per- formance indicators illustrates that one measure of performance may not be sufficient to accurately pre- 1. -

ESTADÍSTICO DE PESCA Índice
ANUARIO ESTADÍSTICO DE PESCA Índice INTRODUCCIÓN 7 CAPÍTULO I PRODUCCIÓN PESQUERA 13 CAPÍTULO II INDUSTRIALIZACIÓN 109 CAPÍTULO III COMERCIALIZACIÓN Y CONSUMO 123 CAPÍTULO IV FACTORES DE PRODUCCIÓN 147 CAPÍTULO V NORMATIVIDAD 177 CAPÍTULO VI ESTADÍSTICAS INTERNACIONALES 201 GLOSARIO 237 ÍNDICE DE CUADROS 243 ANEXO 257 SECRETARÍA DE AGRICULTURA, GANADERÍA, DESARROLLO RURAL, PESCA Y ALIMENTACIÓN Javier Bernardo Usabiaga Arroyo SECRETARIO Jerónimo Ramos Sáenz Pardo COMISIONADO NACIONAL DE ACUACULTURA Y PESCA Juan Carlos Cortés García SUBSECRETARIO DE PLANEACIÓN Víctor Villalobos Arámbula SUBSECRETARIO DE AGRICULTURA Y GANADERÍA Antonio Ruiz García SUBSECRETARIO DE DESARROLLO RURAL Mara Angélica Murillo Correa DIRECTORA GENERAL DE POLÍTICA Y FOMENTO PESQUERO Guillermo Compean Jiménez PRESIDENTE DEL INSTITUTO NACIONAL DE LA PESCA p Introducción Introducción a Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación, tiene como uno de sus propósitos esenciales difundir en forma confiable y oportuna, los L principales indicadores de la actividad pesquera en México, que son importantes para conocer el comportamiento y evolución de la explotación, conservación e industrialización de la flora y fauna acuática del país. La SAGARPA a través del desarrollo y actualización de su infraestructura informática y el rediseño de los sistemas estadísticos, aunado a la automatización en sus procesos, propicia las condiciones necesarias para la generación de información estadística actual y confiable, que permite conocer los fenómenos que comprende la pesca en su conjunto. Para la integración de este documento fue necesaria una cercana vinculación entre las delegaciones federales, las oficinas de la SAGARPA y los órganos centrales de la Secretaría, quienes por medio de procedimiento ya establecido, llevaron a cabo la tarea de recopilar e integrar la información estadística emanada de los diferentes agentes que participan activamente en este sector. -

Phylogenetic Evidence from Freshwater Crayfishes That Cave Adaptation Is Not an Evolutionary Dead- End David B
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by DigitalCommons@Florida International University Florida International University FIU Digital Commons Center for Coastal Oceans Research Faculty Institute of Water and Enviornment Publications 9-20-2017 Phylogenetic evidence from freshwater crayfishes that cave adaptation is not an evolutionary dead- end David B. Stern George Washington University Jesse Breinholt University of Florida Carlos Pedrazaz-Lara Universidad Nacional Autónoma de México Marilu Lopez-Mejia Universidad de Quintana Roo Christopher L. Owen George Washington University See next page for additional authors Follow this and additional works at: https://digitalcommons.fiu.edu/merc_fac Part of the Life Sciences Commons Recommended Citation Stern, David B.; Breinholt, Jesse; Pedrazaz-Lara, Carlos; Lopez-Mejia, Marilu; Owen, Christopher L.; Bracken-Grissom, Heather D.; Fetzner, James W. Jr.; and Crandall, Keith A., "Phylogenetic evidence from freshwater crayfishes that cave adaptation is not an evolutionary dead-end" (2017). Center for Coastal Oceans Research Faculty Publications. 26. https://digitalcommons.fiu.edu/merc_fac/26 This work is brought to you for free and open access by the Institute of Water and Enviornment at FIU Digital Commons. It has been accepted for inclusion in Center for Coastal Oceans Research Faculty Publications by an authorized administrator of FIU Digital Commons. For more information, please contact [email protected]. Authors David B. Stern, Jesse Breinholt, Carlos Pedrazaz-Lara, Marilu Lopez-Mejia, Christopher L. Owen, Heather D. Bracken-Grissom, James W. Fetzner Jr., and Keith A. Crandall This article is available at FIU Digital Commons: https://digitalcommons.fiu.edu/merc_fac/26 BRIEF COMMUNICATION doi:10.1111/evo.13326 Phylogenetic evidence from freshwater crayfishes that cave adaptation is not an evolutionary dead-end David B. -

Taxonomy, Biology and Distribution of Lobsters
Taxonomy, Biology and Distribution of Lobsters 15 Rekha Devi Chakraborty and E.V.Radhakrishnan Crustacean Fisheries Division, Central Marine Fisheries Research Institute, Kochi-682 018 Lobsters are among the most prized of fisheries resources and of significant commercial interest in many countries. Because of their high value and esteemed culinary worth, much attention has been paid to lobsters in biological, fisheries, and systematic literature. They have a great demand in the domestic market as a delicacy and is a foreign exchange earner for the country. Taxonomic status Phylum: Arthropoda Subphylum: Crustacea Class: Malacostraca Subclass: Eumalacostraca Superorder: Eucarida Order: Decapoda Suborder: Macrura Reptantia The suborder Macrura Reptantia consists of three infraorders: Astacidea (Marine lobsters and freshwater crayfishes), Palinuridea (Spiny lobsters and slipper lobsters) and Thalassinidea (mud lobsters). The infraorder Astacidea Summer School on Recent Advances in Marine Biodiversity Conservation and Management 100 Rekha Devi Chakraborty and E.V.Radhakrishnan contains three superfamilies of which only one (the Infraorder Palinuridea, Superfamily Eryonoidea, Family Nephropoidea) is considered here. The remaining two Polychelidae superfamilies (Astacoidea and parastacoidea) contain the 1b. Third pereiopod never with a true chela,in most groups freshwater crayfishes. The superfamily Nephropoidea (40 chelae also absent from first and second pereiopods species) consists almost entirely of commercial or potentially 3a Antennal flagellum reduced to a single broad and flat commercial species. segment, similar to the other antennal segments ..... Infraorder Palinuridea, Superfamily Palinuroidea, The infraorder Palinuridea also contains three superfamilies Family Scyllaridae (Eryonoidea, Glypheoidea and Palinuroidea) all of which are 3b Antennal flagellum long, multi-articulate, flexible, whip- marine. The Eryonoidea are deepwater species of insignificant like, or more rigid commercial interest. -

Large Spiny Lobsters Reduce the Catchability of Small Conspecifics
Vol. 666: 99–113, 2021 MARINE ECOLOGY PROGRESS SERIES Published May 20 https://doi.org/10.3354/meps13695 Mar Ecol Prog Ser OPEN ACCESS Size matters: large spiny lobsters reduce the catchability of small conspecifics Emma-Jade Tuffley1,2,3,*, Simon de Lestang2, Jason How2, Tim Langlois1,3 1School of Biological Sciences, The University of Western Australia, 35 Stirling Highway, Crawley, WA 6009, Australia 2Aquatic Science and Assessment, Department of Primary Industries and Regional Development, 39 Northside Drive, Hillarys, WA 6025, Australia 3The UWA Oceans Institute, Indian Ocean Marine Research Centre, Cnr. of Fairway and Service Road 4, Crawley, WA 6009, Australia ABSTRACT: Indices of lobster abundance and population demography are often derived from pot catch rate data and rely upon constant catchability. However, there is evidence in clawed lobsters, and some spiny lobsters, that catchability is affected by conspecifics present in pots, and that this effect is sex- and size-dependent. For the first time, this study investigated this effect in Panulirus cyg nus, an economically important spiny lobster species endemic to Western Australia. Three studies: (1) aquaria trials, (2) pot seeding experiments, and (3) field surveys, were used to investi- gate how the presence of large male and female conspecifics influence catchability in smaller, immature P. cygnus. Large P. cygnus generally reduced the catchability of small conspecifics; large males by 26−33% and large females by 14−27%. The effect of large females was complex and varied seasonally, dependent on the sex of the small lobster. Conspecific-related catchability should be a vital consideration when interpreting the results of pot-based surveys, especially if population demo graphy changes. -

REMINDER: Supporting Seafood Future (SSF) Grants Program
PFA Update 4 January 2019 The PFA represents its members’ interests. If you need our help on any issue, please do not hesitate in contacting the PFA head office (6652-7374) &/or the Chief Executive Officer (0429303371) REMINDER: Supporting Seafood Future (SSF) grants program The Supporting Seafood Future (SSF) grants program is designed to build marketing and promotion capability within seafood businesses through small-scale (less than $10,000 including GST) and large-scale ($10,000 to $200,000 including GST) grants. The purpose of the campaign is to increase consumption of NSW seafood, drive the value of NSW seafood through increased awareness and consumption, and build industry capabilities and cohesiveness. Strategic objectives of the campaign are to: • Increase value, through consumption of farmed and wild caught NSW seafood • Increase shopper options across a broader variety of NSW seafood • Drive awareness and build social licence of the NSW seafood community Contact the PFA if you have any ideas that you wish our support, partnership or would like us to help put together an application. Information regarding program guidelines are outlined in the Program Guidelines. NATIONAL SEAFOOD INDUSTRY LEADERSHIP PROGRAM (NSILP 2019) Applications are now open for the 2019 National Seafood Industry Leadership Program. Each year the Sydney Fish Market is proud to fund 2-3 staff/industry representatives to attend the program. If you would like to be put forward by SFM as one of their sponsored candidates, please contact SFM or the PFA for the nomination form. These forms must be returned to SFM by Friday Jan 11th. The National Seafood Industry Leadership Program (NSLIP) is designed for people wishing to take up leadership roles throughout the industry. -

104-111, March-June 1993
Indian Journal of Fisheries 40 (1,2) : 104-111, March-June 1993 Crustacean fishery resources of India - An overview C SUSEELAN' and N N PILLAI^ Central Marine Fisheries Research Institute, Kochin, Kerala 682 014 ABSTRACT The recent trend in crustacean fishery of India has been reviewed based on the landings during 1984-1992. The fishery as a \^iiole inqjroved over the years reaching a record level of 0.39 miUicn tcones in 1991. Brawn landings, which accounted for about 72% of the crustacean fishery, showed a reaunkable leap since 1988 as a result of extended fishing by shrimp trawlers over time and ^ace. and the innovative fishing in the traditional sectcr. The changed fishing pattern in the mechanized as well as aitisanal sectors, and its intact en catch and ^ecies conq)osition are outlined. The average production of 0.225 millioo tcnnes of prawns realized at present is very close to the estimated catdiable potential of the 0-50 m depth zone. Stock assessments of in:;>ortant species also reveals that the coastal >shrinq> resource of India is fiilly e;q>loited at present. Some species like Metapenaeus dohsoni, Penaeus indicus and P. semisulcatus ate overfished in their respective areas of fishery. The lobster fishery, on the whole, is in a state of decline. On the north-west coast, stock assessment of the princ^al ^ecies Panulirus polyphagus has shown that to reach the MSY level trawling e£foit would have to be considerably reduced which may not be feasible as this fishing e£foit is targeted for other resources. -

SPINY LOBSTER AMENDMENT 10 I ACRONYMS/ABBREVIATIONS
DRAFT Amendment 10 to the Fishery Management Plan for Spiny Lobster in the Gulf of Mexico and South Atlantic with Draft Environmental Impact Statement, Initial Regulatory Flexibility Act Analysis, Regulatory Impact Review, and Social Impact Assessment/Fishery Impact Statement June 2010 1 Gulf of Mexico Fishery Management Council South Atlantic Fishery Management Council 2203 N. Lois Avenue, Suite 1100 4055 Faber Place Drive, Suite 201 Tampa, FL 33607 North Charleston, South Carolina 29405 (813) 348-1630 (Phone) (843) 571-4366 (Phone) (888) 833-1844 (Toll Free) (866) safmc-10 (Toll Free) (813) 348-1711 (Fax) (843) 769-4520 (Fax) Website: www.gulfcouncil.org Email (general): [email protected] Website: www.safmc.net National Oceanic & Atmospheric Administration National Marine Fisheries Service Southeast Regional Office 263 13th Avenue South St. Petersburg, Florida 33701 727-824-5308 727-824-5305 (fax) http://sero.nmfs.noaa.gov This is a publication of the Gulf of Mexico Fishery Management Council Pursuant to National Oceanic and Atmospheric Administration Award No. NA10NMF4410011 and the South Atlantic Fishery Management Council Pursuant to NOAA Award No. FNA05NMF4410004 Acronyms/Abbreviations ABC acceptable biological catch ACL annual catch limit ACOE Army Corps of Engineers ACT annual catch target ADCNR, MRD Alabama Department of Conservation and Natural Resources, Marine Resources Division AM accountability measure APA Administrative Procedure Act AP advisory panel ASMFC Atlantic States Marine Fisheries Commission B Biomass BCURRENT current biomass of stock BMSY Biomass at MSY CEQ Council on Environmental Quality CFMC Caribbean Fishery Management Council CFR Code of Federal Regulations Councils Gulf of Mexico Fishery and South Atlantic Management Councils CPUE catch per unit effort CL Carapace Length CSL Caribbean Spiny Lobster CWA Clean Water Act CZMA Coastal Zone Management Act DEIS draft environmental impact statement DOC U. -

Marine Policy Marine Aquarium Trade in India
Marine Policy 77 (2017) 120–129 Contents lists available at ScienceDirect Marine Policy journal homepage: www.elsevier.com/locate/marpol Marine aquarium trade in India: Challenges and opportunities for MARK conservation and policy ⁎ Sanjeevi Prakasha, , Thipramalai Thangappan Ajith Kumarb, Rajeev Raghavanc, Andrew Rhyned, Michael F. Tlustye,f,g, Thanumalaya Subramoniama a Centre for Climate Change Studies, Sathyabama University, Rajiv Gandhi Salai, Chennai 600 119, Tamil Nadu, India b National Bureau of Fish Genetic Resources (ICAR), Canal Ring Road, Dilkusha Post, Lucknow 226 002, Uttar Pradesh, India c Department of Fisheries Resource Management, Kerala University of Fisheries and Ocean Studies (KUFOS), Panangad, Kochi 682 506, Kerala, India d Department of Biology and Marine Biology, Roger Williams University, Bristol, RI, USA e Anderson Cabot Center for Ocean Life, Boston, MA, USA f New England Aquarium, Boston, MA, USA g University of Massachusetts Boston, Boston, MA, USA ARTICLE INFO ABSTRACT Keywords: The collection of marine taxa for the aquarium trade continues to demand live animals be extracted from reefs, Aquarium trade but in doing so, offers economic benefits for local communities. To improve our understanding of the status of Gulf of Mannar marine aquariumtrade in India, information on harvested species and their volume was gathered at the major IUCN Red List collection hubs (Tuticorin, Kilakarai and Mandapam) in the Gulf of Mannar region, and compared to the export Market discrepancy data. During one year, 87 species of fish (51% belonging to the family Pomacentridae) and 21 species of invertebrates were harvested for the trade. The conservation status of exploited species revealed that nearly 50% (n=43) have not been assessed for their extinction risk by the IUCN, while of the 44 species assessed, 41 were Least Concern (LC), and one each was in the Data Deficient (DD), Near Threatened (NT) and Endangered (EN) categories.