The Status of Queensland's Moreton Bay Bug (Thenus Spp.) And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

U , '' Regional (;"Ntre of I:::;~, I Central Marine Fisheries Research Institute T - ~ '\~ ~ ~3L'!" ICAR Marine Fisheries P.O
CMFRI S ammelt S ,4(J(Jt (JU Recent Advances in Se"ed Production and Growout Techniques for Marine Finfish and Shellfish ,1 I Compiled and Edited by Dr. G.Gopakumar, Principal Scientist & Director, Summer School and '. Dr. Boby Ignatius, Scientist (SS) Central Marine Fisheries Research Institute j' U , '' Regional (;"ntre of i:::;~, I Central Marine Fisheries Research Institute t - ~ '\~ ~ ~3l'!" ICAR Marine Fisheries P.O . Tamil Nadu - 623 520 ' ~~ ... ~"'~~ SEED PRODUCTION OF THE SAND LOBSTER THENUS ORIENTALIS (LUND) Joe K. Kizhakudan Research Centre of CMFRI, Chennai 3f With the decline in many commercial fisheries worldwide and an ever increasing demand for seafood protein, there is a growing need for augmenting the production of high-protein, high-value resources like lobsters. Aquaculture remains the ideal measure to augment production and ensure conseNation, and even enhancement. of natural stocks. Aquaculture provides a two-pronged solution towards increasing the fish production through ., farming of hatchery-produced seed of commercially important finfishes and shellfishes , enhancing natural stocks by sea ranching hatchery-produced seed of commercially important finfishes and shellfishes Lobsters are among the most priced seafood delicacies enjoying a special demand in international markets. As against a world average annual productio'n of2.1 lakh tonnes, India's average annual lobster production is about 2000 tonnes. With the distinction of being perhaps, the only seafood resource in India's trade economy, which remains relatively low down the ladder in terms of quantity of production but brings in maximum foreign exchange, lobsters have been the subject of study for more than two decades now. -

Lobsters-Identification, World Distribution, and U.S. Trade
Lobsters-Identification, World Distribution, and U.S. Trade AUSTIN B. WILLIAMS Introduction tons to pounds to conform with US. tinents and islands, shoal platforms, and fishery statistics). This total includes certain seamounts (Fig. 1 and 2). More Lobsters are valued throughout the clawed lobsters, spiny and flat lobsters, over, the world distribution of these world as prime seafood items wherever and squat lobsters or langostinos (Tables animals can also be divided rougWy into they are caught, sold, or consumed. 1 and 2). temperate, subtropical, and tropical Basically, three kinds are marketed for Fisheries for these animals are de temperature zones. From such partition food, the clawed lobsters (superfamily cidedly concentrated in certain areas of ing, the following facts regarding lob Nephropoidea), the squat lobsters the world because of species distribu ster fisheries emerge. (family Galatheidae), and the spiny or tion, and this can be recognized by Clawed lobster fisheries (superfamily nonclawed lobsters (superfamily noting regional and species catches. The Nephropoidea) are concentrated in the Palinuroidea) . Food and Agriculture Organization of temperate North Atlantic region, al The US. market in clawed lobsters is the United Nations (FAO) has divided though there is minor fishing for them dominated by whole living American the world into 27 major fishing areas for in cooler waters at the edge of the con lobsters, Homarus americanus, caught the purpose of reporting fishery statis tinental platform in the Gul f of Mexico, off the northeastern United States and tics. Nineteen of these are marine fish Caribbean Sea (Roe, 1966), western southeastern Canada, but certain ing areas, but lobster distribution is South Atlantic along the coast of Brazil, smaller species of clawed lobsters from restricted to only 14 of them, i.e. -
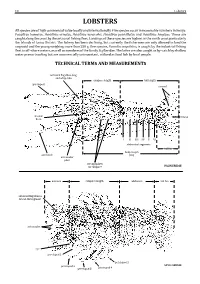
Lobsters LOBSTERS§
18 Lobsters LOBSTERS§ All species are of high commercial value locally and internationally. Five species occur in reasonable numbers in Kenya: Panulirus homarus, Panulirus ornatus, Panulirus versicolor, Panulirus penicillatus and Panulirus longipes. These are caughtungravid along and the the coast young by weighing the artisanal more fishing than 250 fleet. g. Landings One species, of these Puerulus species angulatus are highest in the north coast particularly the Islands of Lamu District. The fishery has been declining,Scyllaridae. but currently The latter the fishermen are also caught are only as by–catch allowed toby landshallow the , is caught by the industrial fishing fleet in off–shore waters, as well as members of the family water prawn trawling but areTECHNICAL commercially unimportant, TERMS AND utilized MEASUREMENTS as food fish by local people. and whip–like antennal flagellum long carapace length tail length pereiopod uropod frontal telson horn III III IV VIV abdominal segments tail fan body length antennule (BL) antennular plate strong spines on carapace PALINURIDAE antenna carapace length abdomen tail fan antennal flagellum a broad, flat segment antennules eye pereiopod 1 pereiopod 5 pereiopod 2 SCYLLARIDAE pereiopod 3 pereiopod 4 Guide to Families 19 GUIDE TO FAMILIES NEPHROPIDAE Page 20 True lobsters § To about 15 cm. Marine, mainly deep waters on soft included in the Guide to Species. 1st pair of substrates. Three species of interest to fisheriespereiopods are large 3rd pair of pereiopods with chela PALINURIDAE Page 21 Antennal Spiny lobsters § To about 50 cm. Marine, mostly shallow waters on flagellum coral and sand stone reefs, some species on soft included in the Guide to Species. -

Does Climate Change Bolster the Case for Fishery Reform in Asia? Christopher Costello∗
Does Climate Change Bolster the Case for Fishery Reform in Asia? Christopher Costello∗ I examine the estimated economic, ecological, and food security effects of future fishery management reform in Asia. Without climate change, most Asian fisheries stand to gain substantially from reforms. Optimizing fishery management could increase catch by 24% and profit by 34% over business- as-usual management. These benefits arise from fishing some stocks more conservatively and others more aggressively. Although climate change is expected to reduce carrying capacity in 55% of Asian fisheries, I find that under climate change large benefits from fishery management reform are maintained, though these benefits are heterogeneous. The case for reform remains strong for both catch and profit, though these numbers are slightly lower than in the no-climate change case. These results suggest that, to maximize economic output and food security, Asian fisheries will benefit substantially from the transition to catch shares or other economically rational fishery management institutions, despite the looming effects of climate change. Keywords: Asia, climate change, fisheries, rights-based management JEL codes: Q22, Q28 I. Introduction Global fisheries have diverged sharply over recent decades. High governance, wealthy economies have largely adopted output controls or various forms of catch shares, which has helped fisheries in these economies overcome inefficiencies arising from overfishing (Worm et al. 2009) and capital stuffing (Homans and Wilen 1997), and allowed them to turn the corner toward sustainability (Costello, Gaines, and Lynham 2008) and profitability (Costello et al. 2016). But the world’s largest fishing region, Asia, has instead largely pursued open access and input controls, achieving less long-run fishery management success (World Bank 2017). -

Eucrustacea: Decapoda: Scyllaridae) from the Eocene of Pakistan Denis Audo
First occurrence of Ibacinae (Eucrustacea: Decapoda: Scyllaridae) from the Eocene of Pakistan Denis Audo To cite this version: Denis Audo. First occurrence of Ibacinae (Eucrustacea: Decapoda: Scyllaridae) from the Eocene of Pakistan. Journal of Systematic Palaeontology, Taylor & Francis, 2019, 17 (6), pp.533-538. 10.1080/14772019.2017.1405085. hal-02274560 HAL Id: hal-02274560 https://hal.sorbonne-universite.fr/hal-02274560 Submitted on 30 Aug 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. First occurrence of Ibacinae (Eucrustacea: Decapoda: Scyllaridae) from the Eocene of Pakistan Denis Audoa, b aUMR CNRS 6118 Géosciences, Université de Rennes I, Campus de Beaulieu, avenue du général Leclerc, 35042 Rennes cedex, France. E-mail: [email protected] bMuséum national d’Histoire naturelle, Centre de Recherche sur la Paléobiodiversité et les Paléoenvironnements (CR2P, UMR 7207), Sorbonne Universités, MNHN, UPMC, CNRS, 57 rue Cuvier F-75005 Paris, France. Abstract The fossil record of slipper lobsters (Scyllaridae) is very poor, even though the earliest species of the modern slipper lobsters (Neoscyllaridae) are known from the Early Cretaceous. Among the clades “subfamilies” of slipper lobsters, perhaps the most remarkable is Ibacinae (currently monogeneric), which contains species with a very wide carapace (cephalothoracic shield) and very deep incisions. -

Goldstein Et Al 2019
Journal of Crustacean Biology Advance Access published 24 August 2019 Journal of Crustacean Biology The Crustacean Society Journal of Crustacean Biology 39(5), 574–581, 2019. doi:10.1093/jcbiol/ruz055 Downloaded from https://academic.oup.com/jcb/article-abstract/39/5/574/5554142/ by University of New England Libraries user on 04 October 2019 Development in culture of larval spotted spiny lobster Panulirus guttatus (Latreille, 1804) (Decapoda: Achelata: Palinuridae) Jason S. Goldstein1, Hirokazu Matsuda2, , Thomas R. Matthews3, Fumihiko Abe4, and Takashi Yamakawa4, 1Wells National Estuarine Research Reserve, Maine Coastal Ecology Center, 342 Laudholm Farm Road, Wells, ME 04090 USA; 2Mie Prefecture Fisheries Research Institute, 3564-3, Hamajima, Shima, Mie 517-0404 Japan; 3Florida Fish and Wildlife Conservation Commission, Fish and Wildlife Research Institute, 2796 Overseas Hwy, Suite 119, Marathon, FL 33050 USA; and 4Department of Aquatic Bioscience, Graduate School of Agricultual and Life Sciences, The University of Tokyo, 1-1-1 Yayoi, Bunkyo, Tokyo 113-8657 Japan HeadA=HeadB=HeadA=HeadB/HeadA Correspondence: J.S. Goldstein: e-mail: [email protected] HeadB=HeadC=HeadB=HeadC/HeadB (Received 15 May 2019; accepted 11 July 2019) HeadC=HeadD=HeadC=HeadD/HeadC Ack_Text=DisHead=Ack_Text=HeadA ABSTRACT NList_lc_rparentheses_roman2=Extract1=NList_lc_rparentheses_roman2=Extract1_0 There is little information on the early life history of the spotted spiny lobster Panulirus guttatus (Latreille, 1804), an obligate reef resident, despite its growing importance as a fishery re- BOR_HeadA=BOR_HeadB=BOR_HeadA=BOR_HeadB/HeadA source in the Caribbean and as a significant predator. We cultured newly-hatched P. guttatus BOR_HeadB=BOR_HeadC=BOR_HeadB=BOR_HeadC/HeadB larvae (phyllosomata) in the laboratory for the first time, and the growth, survival, and mor- BOR_HeadC=BOR_HeadD=BOR_HeadC=BOR_HeadD/HeadC phological descriptions are reported through 324 days after hatch (DAH). -

The Biology and Fisheries of the Slipper Lobster
The Biology and Fisheries of the Slipper Lobster Kari L. Lavalli College of General Studies Boston University Bo~ton, Massachusetts, U.S.A. Ehud Spanier The Leon Recanati Institute for Maritime Studies and Department of Maritime Civilizations University of Haifa Haifa, Israel 0 ~y~~F~~~~~oup Boca Raton London New York CRC Press is an imprint of the Taylor & Francis Group, an informa business Cover image courtesy of Megan Elizabeth Stover of the College of General Studies, Boston University, Boston, Massachusetts. CRC Press Taylor & Francis Group 6000 Broken Sound Parkway NW, Suite 300 Boca Raton, FL 33487-2742 © 2007 by Taylor & Francis Group, LLC CRC Press is an imprint of Taylor & Francis Group, an Informa business No claim to original U.S. Government works Printed in the United States of America on acid-free paper 10 9 8 7 6 5 4 3 2 1 International Standard Book Number-10: 0-8493-3398-9 (Hardcover) International Standard Book Number-13: 978-0-8493-3398-9 (Hardcover) This book contains information obtained from authentic and highly regarded sources. Reprinted material is quoted with permission, and sources are indicated. A wide variety of references are listed. Reasonable efforts have been made to publish reliable data and information, but the author and the publisher cannot assume responsibility for the validity of all materials or for the consequences of their use. No part ofthis book may be reprinted, reproduced, transmitted, or utilized in any form by any electronic, mechanical, or other means, now known or hereafter invented, including photocopying, microfilming, and recording, or in any informa tion storage or retrieval system, without written permission from the publishers. -

Taxonomy, Biology and Distribution of Lobsters
Taxonomy, Biology and Distribution of Lobsters 15 Rekha Devi Chakraborty and E.V.Radhakrishnan Crustacean Fisheries Division, Central Marine Fisheries Research Institute, Kochi-682 018 Lobsters are among the most prized of fisheries resources and of significant commercial interest in many countries. Because of their high value and esteemed culinary worth, much attention has been paid to lobsters in biological, fisheries, and systematic literature. They have a great demand in the domestic market as a delicacy and is a foreign exchange earner for the country. Taxonomic status Phylum: Arthropoda Subphylum: Crustacea Class: Malacostraca Subclass: Eumalacostraca Superorder: Eucarida Order: Decapoda Suborder: Macrura Reptantia The suborder Macrura Reptantia consists of three infraorders: Astacidea (Marine lobsters and freshwater crayfishes), Palinuridea (Spiny lobsters and slipper lobsters) and Thalassinidea (mud lobsters). The infraorder Astacidea Summer School on Recent Advances in Marine Biodiversity Conservation and Management 100 Rekha Devi Chakraborty and E.V.Radhakrishnan contains three superfamilies of which only one (the Infraorder Palinuridea, Superfamily Eryonoidea, Family Nephropoidea) is considered here. The remaining two Polychelidae superfamilies (Astacoidea and parastacoidea) contain the 1b. Third pereiopod never with a true chela,in most groups freshwater crayfishes. The superfamily Nephropoidea (40 chelae also absent from first and second pereiopods species) consists almost entirely of commercial or potentially 3a Antennal flagellum reduced to a single broad and flat commercial species. segment, similar to the other antennal segments ..... Infraorder Palinuridea, Superfamily Palinuroidea, The infraorder Palinuridea also contains three superfamilies Family Scyllaridae (Eryonoidea, Glypheoidea and Palinuroidea) all of which are 3b Antennal flagellum long, multi-articulate, flexible, whip- marine. The Eryonoidea are deepwater species of insignificant like, or more rigid commercial interest. -

Historic Naturalis Classica, Viii Historic Naturalis Classica
HISTORIC NATURALIS CLASSICA, VIII HISTORIC NATURALIS CLASSICA EDIDERUNT J. CRAMER ET H.K.SWANN TOMUS vm BIBUOGRAPHY OF THE LARVAE OF DECAPOD CRUSTACEA AND LARVAE OF DECAPOD CRUSTACEA BY ROBERT GURNEY WITH 122 FIGURES IN THE TEXT REPRINTED 1960 BY H. R. ENGELMANN (J. CRAMER) AND WHELDON & WESLEY, LTD. WEINHEIM/BERGSTR. CODICOTE/HERTS. BIBLIOGRAPHY OF THE LARVAE OF DECAPOD CRUSTACEA AND LARVAE OF DECAPOD CRUSTACEA BY ROBERT GURNEY WITH 122 FIGURES IN THE TEXT REPRINTED 1960 BY H. R. ENGELMANN (J. CRAMER) AND WHELDON & WESLEY, LTD. WEINHEIM/BERGSTR. CODICOTE/HERTS. COPYRIGHT 1939 & 1942 BY THfi RAY SOCIETY IN LONDON AUTHORIZED REPRINT COPYRIGHT OF THE SERIES BY J. CRAMER PUBLISHER IN WEINHEIM PRINTED IN GERMANY I9«0 i X\ T • THE RAY SOCIETY INSTITUTED MDCCCXLIV This volume (No. 125 of the Series) is issued to the Svhscribers to the RAY SOCIETY JOT the Year 1937. LONDON MCMXXXIX BIBLIOGKAPHY OF THE LARVAE OF DECAPOD CRUSTACEA BY ROBERT GURNEY, M.A., D.Sc, F.L.S. LONDON PRINTED FOR THE RAT SOCIETY SOLD BT BERNARD QUARITCH, LTD. U, GBAFTOK STBKET, NBW BOND STEBBT, LONDON, "W. 1 1939 PRINTED BY ADLABD AND SON, LIMITED 2 1 BLOOJlSBUBY WAY, LONDON, W.C. I Madt and printed in Great Britain. CONTENTS PAOE PBBFACE . " V BiBUOGRAPHY CLASSIFIED LIST . 64 Macrura Natantia 64 Penaeidea 64 Caridea 70 Macrura Reptantia 84 Nephropsidea 84 Eryonidea 88 Scyllaridea 88 Stenopidea 91 Thalassinidea 92 Anomura ; 95 Galatheidea . 95 Paguridea 97 Hippidea 100 Dromiacea 101 Brachyura 103 Gymnopleura 103 Brachygnatha 103 Oxyrhyncha 113 Oxystomata . 116 INDEX TO GENERA 120 PREFACE IT has been my intention to publish a monograph of Decapod larvae which should contain a bibliography, a part dealing with a number of general questions relating to the post-embryonic development of Decapoda and Euphausiacea, and a series of sections describing the larvae of all the groups, so far as they are known. -

Developmental Changes in the Mouthparts of Juvenile Caribbean Spiny Lobster, Panulirus Argus: Implications for Aquaculture
FAD LIBRARIES Florida Atlantic University HARBOR BRANCH ~ FLORIDA ATLANTIC UNIVERSITY FAU Institutional Repository http://purl.fcla.edu/fau/fauir This paper was submitted by the faculty of FAU’s Harbor Branch Oceanographic Institute. Notice: ©2008 Elsevier B.V. The final published version of this manuscript is available at http://www.sciencedirect.com/science/journal/00448486. This article may be cited as: Cox, S. L., Jeffs, A. G., & Davis, M. (2008). Developmental changes in the mouthparts of juvenile Caribbean spiny lobster, Panulirus argus: Implications for aquaculture. Aquaculture, 283(1‐4), 168‐174. doi:10.1016/j.aquaculture.2008.07.019 Aquaculture 283 (2008) 168–174 Contents lists available at ScienceDirect Aquaculture journal homepage: www.elsevier.com/locate/aqua-online Developmental changes in the mouthparts of juvenile Caribbean spiny lobster, Panulirus argus: Implications for aquaculture Serena L. Cox a,b,⁎, Andrew G. Jeffs c, Megan Davis a a Aquaculture Division, Harbor Branch Oceanographic Institute at Florida Atlantic University, 5600 US 1 North, Fort Pierce, Florida 34946, USA b National Institute of Water and Atmospheric Research, Private Bag 14901, Kilbirnie, Wellington 6241, New Zealand c Leigh Marine Laboratory, University of Auckland, P.O. Box 349, Warkworth 0941, New Zealand article info abstract Article history: Light microscopy and video analysis were used to examine the mouthpart morphology and feeding Received 5 May 2008 behaviour of the Caribbean spiny lobster from puerulus (megalopal stage) (5–8 mm carapace length, CL) to Received in revised form 14 July 2008 adult (85 mm CL). Upon settlement the pueruli did not possess fully functional mouthparts, however, Accepted 14 July 2008 efficient feeding appendages appeared in the first instar juvenile (after the first moult from puerulus). -

Slipper Lobsters (Scyllaridae) Off the Southeastern Coast of Brazil: Relative Growth, Population Structure, and Reproductive
55 Abstract—The hooded slipper lobster Slipper lobsters (Scyllaridae) off the (Scyllarides deceptor) and Brazilian slipper lobster (S. brasiliensis) are southeastern coast of Brazil: relative growth, commonly caught by fishing fleets (with double-trawling and longline population structure, and reproductive biology pots and traps) off the southeastern coast of Brazil. Their reproductive Luis Felipe de Almeida Duarte (contact author)1 biology is poorly known and research 2 on these 2 species would benefit ef- Evandro Severino-Rodrigues forts in resource management. This Marcelo A. A. Pinheiro3 study characterized the population Maria A. Gasalla4 structure of these exploited species on the basis of sampling from May Email address for contact author: [email protected] 2006 to April 2007 off the coast of Santos, Brazil. Data for the abso- 1 Departamento de Zoologia 3 Laboratório de Biologia de Crustáceos lute fecundity, size at maturity in Ca[mpus de Rio Claro Grupo de Pesquisa em Biologia de Crustáceos females, reproductive period, and Universidade Estadual Paulista Ca[mpus Experimental do Litoral Paulista morphometric relationships of the Avenida 24 A, 1515 Universidade Estadual Paulista dominant species, the hooded slipper 13506-900, Rio Claro Praça Infante D. Henrique lobster, are presented. Significant São Paulo, Brazil s/n°11330-900, São Vicente differential growth was not observed 2 São Paulo, Brazil between juveniles and adults of each Instituto de Pesca 4 sex, although there was a small in- Agência Paulista de Tecnologia dos Laboratório de Ecossistemas Pesqueiros vestment of energy in the width and Agronegócios Departamento de Oceanográfico Biológica length of the abdomen in females Secretaria de Agricultura e Abastecimento Instituto Oceanográfico and in the carapace length for males Governo do Estado São Paulo Universidade de São Paulo in larger animals (>25 cm in total Avenida Bartolomeu de Gusmão, 192 Praça do Oceanográfico, 191 length [TL]). -

Digging Mechanisms and Substrate Preferences of Shovel Nosed Lobsters, Ibacus Peronii (Decapoda: Scyllaridae)
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Scholarworks@UTRGV Univ. of Texas RioGrande Valley University of Texas Rio Grande Valley ScholarWorks @ UTRGV Biology Faculty Publications and Presentations College of Sciences 1-2006 Digging Mechanisms and Substrate Preferences of Shovel Nosed Lobsters, Ibacus peronii (Decapoda: Scyllaridae) Zen Faulkes The University of Texas Rio Grande Valley, [email protected] Follow this and additional works at: https://scholarworks.utrgv.edu/bio_fac Part of the Biology Commons Recommended Citation Faulkes, Zen, "Digging Mechanisms and Substrate Preferences of Shovel Nosed Lobsters, Ibacus peronii (Decapoda: Scyllaridae)" (2006). Biology Faculty Publications and Presentations. 34. https://scholarworks.utrgv.edu/bio_fac/34 This Article is brought to you for free and open access by the College of Sciences at ScholarWorks @ UTRGV. It has been accepted for inclusion in Biology Faculty Publications and Presentations by an authorized administrator of ScholarWorks @ UTRGV. For more information, please contact [email protected], [email protected]. JOURNAL OF CRUSTACEAN BIOLOGY, 26(1): 69–72, 2006 DIGGING MECHANISMS AND SUBSTRATE PREFERENCES OF SHOVEL NOSED LOBSTERS, IBACUS PERONII (DECAPODA: SCYLLARIDAE) Zen Faulkes Department of Zoology, University of Melbourne, Royal Parade, Parkville, Victoria, 3010, Australia; Present address: Department of Biology, University of Texas – Pan American, 1201 W. University Drive, Edinburg, Texas, 78541, U.S.A. ([email protected]) ABSTRACT Digging is a distinct form of locomotion that poses different mechanical problems than other locomotor modes that are commonly used by crustaceans, e.g., walking, swimming. I examined the mechanisms of digging by shovel nosed lobsters (Ibacus peronii), which spend most of the day underneath sand.