Equations of State of Ca-Silicates and Phase Diagram of the Casio3 System Under Upper Mantle Conditions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Download PDF About Minerals Sorted by Mineral Name
MINERALS SORTED BY NAME Here is an alphabetical list of minerals discussed on this site. More information on and photographs of these minerals in Kentucky is available in the book “Rocks and Minerals of Kentucky” (Anderson, 1994). APATITE Crystal system: hexagonal. Fracture: conchoidal. Color: red, brown, white. Hardness: 5.0. Luster: opaque or semitransparent. Specific gravity: 3.1. Apatite, also called cellophane, occurs in peridotites in eastern and western Kentucky. A microcrystalline variety of collophane found in northern Woodford County is dark reddish brown, porous, and occurs in phosphatic beds, lenses, and nodules in the Tanglewood Member of the Lexington Limestone. Some fossils in the Tanglewood Member are coated with phosphate. Beds are generally very thin, but occasionally several feet thick. The Woodford County phosphate beds were mined during the early 1900s near Wallace, Ky. BARITE Crystal system: orthorhombic. Cleavage: often in groups of platy or tabular crystals. Color: usually white, but may be light shades of blue, brown, yellow, or red. Hardness: 3.0 to 3.5. Streak: white. Luster: vitreous to pearly. Specific gravity: 4.5. Tenacity: brittle. Uses: in heavy muds in oil-well drilling, to increase brilliance in the glass-making industry, as filler for paper, cosmetics, textiles, linoleum, rubber goods, paints. Barite generally occurs in a white massive variety (often appearing earthy when weathered), although some clear to bluish, bladed barite crystals have been observed in several vein deposits in central Kentucky, and commonly occurs as a solid solution series with celestite where barium and strontium can substitute for each other. Various nodular zones have been observed in Silurian–Devonian rocks in east-central Kentucky. -

Perovskites: Crystal Structure, Important Compounds and Properties
Perovskites: crystal structure, important compounds and properties Peng Gao GMF Group Meeting 12,04,2016 Solar energy resource PV instillations Global Power Demand Terrestrial sun light To start • We have to solve the energy problem. • Any technology that has good potential to cut carbon emissions by > 10 % needs to be explored aggressively. • Researchers should not be deterred by the struggles some companies are having. • Someone needs to invest in scaling up promising solar cell technologies. Origin And History of Perovskite compounds Perovskite is calcium titanium oxide or calcium titanate, with the chemical formula CaTiO3. The mineral was discovered by Gustav Rose in 1839 and is named after Russian mineralogist Count Lev Alekseevich Perovski (1792–1856).” All materials with the same crystal structure as CaTiO3, namely ABX3, are termed perovskites: Origin And History of Perovskite compounds Very stable structure, large number of compounds, variety of properties, many practical applications. Key role of the BO6 octahedra in ferromagnetism and ferroelectricity. Extensive formation of solid solutions material optimization by composition control and phase transition engineering. A2+ B4+ O2- Ideal cubic perovskite structure (ABO3) Classification of Perovskite System Perovskite Systems Inorganic Halide Oxide Perovskites Perovskites Alkali-halide Organo-Metal Intrinsic Doped Perovskites Halide Perovskites Perovskites A2Cl(LaNb2)O7 Perovskites 1892: 1st paper on lead halide perovskites Structure deduced 1959: Kongelige Danske Videnskabernes -

Ultra-High Pressure Aluminous Titanites in Carbonate-Bearing Eclogites at Shuanghe in Dabieshan, Central China
Ultra-high pressure aluminous titanites in carbonate-bearing eclogites at Shuanghe in Dabieshan, central China D. A. CARSWELL Department of Earth Sciences, University of Sheffield, Sheffield $3 7HF, UK R. N. WILSON Department of Geology, University of Leicester, Leicester LE1 7RH, UK AND M. ZtIAI Institute of Geology, Academia Sinica, P.O. Box 634, Beijing 100029, China Abstract Petrographic features and compositions of titanites in eclogites within the ultra-high pressure metamorphic terrane in central Dabieshan are documented and phase equilibria and thermobarometric implications discussed. Carbonate-bearing eclogite pods in marble at Shuanghe contain primary metamorphic aluminous titanites, with up to 39 mol.% Ca(AI,Fe3+)FSiO4 component. These titanites formed as part of a coesite- bearing eclogite assemblage and thus provide the first direct petrographic evidence that AIFTi_IO_j substitution extends the stability of titanite, relative to futile plus carbonate, to pressures within the coesite stability field. However, it is emphasised that A1 and F contents of such titanites do not provide a simple thermobarometric index of P-T conditions but are constrained by the activity of fluorine, relative to CO2, in metamorphic fluids - as signalled by observations of zoning features in these titanites. These ultra-high pressure titanites show unusual breakdown features developed under more H20-rich amphibolite-facies conditions during exhumation of these rocks. In some samples aluminous titanites have been replaced by ilmenite plus amphibole symplectites, in others by symplectitic intergrowths of secondary, lower AI and F, titanite plus plagioclase. Most other coesite-bearing eclogite samples in the central Dabieshan terrane contain peak assemblage rutile often partly replaced by grain clusters of secondary titanites with customary low AI and F contents. -

Occurrence, Alteration Patterns and Compositional Variation of Perovskite in Kimberlites
975 The Canadian Mineralogist Vol. 38, pp. 975-994 (2000) OCCURRENCE, ALTERATION PATTERNS AND COMPOSITIONAL VARIATION OF PEROVSKITE IN KIMBERLITES ANTON R. CHAKHMOURADIAN§ AND ROGER H. MITCHELL Department of Geology, Lakehead University, 955 Oliver Road, Thunder Bay, Ontario P7B 5E1, Canada ABSTRACT The present work summarizes a detailed investigation of perovskite from a representative collection of kimberlites, including samples from over forty localities worldwide. The most common modes of occurrence of perovskite in archetypal kimberlites are discrete crystals set in a serpentine–calcite mesostasis, and reaction-induced rims on earlier-crystallized oxide minerals (typically ferroan geikielite or magnesian ilmenite). Perovskite precipitates later than macrocrystal spinel (aluminous magnesian chromite), and nearly simultaneously with “reaction” Fe-rich spinel (sensu stricto), and groundmass spinels belonging to the magnesian ulvöspinel – magnetite series. In most cases, perovskite crystallization ceases prior to the resorption of groundmass spinels and formation of the atoll rim. During the final evolutionary stages, perovskite commonly becomes unstable and reacts with a CO2- rich fluid. Alteration of perovskite in kimberlites involves resorption, cation leaching and replacement by late-stage minerals, typically TiO2, ilmenite, titanite and calcite. Replacement reactions are believed to take place at temperatures below 350°C, 2+ P < 2 kbar, and over a wide range of a(Mg ) values. Perovskite from kimberlites approaches the ideal formula CaTiO3, and normally contains less than 7 mol.% of other end-members, primarily lueshite (NaNbO3), loparite (Na0.5Ce0.5TiO3), and CeFeO3. Evolutionary trends exhibited by perovskite from most localities are relatively insignificant and typically involve a decrease in REE and Th contents toward the rim (normal pattern of zonation). -

In Situ X-Ray Diffraction Study of Phase Transitions of Fetio3 at High Pressures and Temperatures Using a Large-Volume Press and Synchrotron Radiation
American Mineralogist, Volume 91, pages 120–126, 2006 In situ X-ray diffraction study of phase transitions of FeTiO3 at high pressures and temperatures using a large-volume press and synchrotron radiation LI CHUNG MING,1,* YOUNG-HO KIM,2 T. UCHIDA,3 Y. WANG,3 AND M. RIVERS3 1Hawaii Institute of Geophysics and Planetology, University of Hawaii, Honolulu, Hawaii 96822, U.S.A. 2Department of Earth and Environment Science, Gyeongsang National University, Jinju 660-701, Korea 3The University of Chicago, 5640 South Ellis Avenue, Chicago, Illinois 60637, U.S.A. ABSTRACT The phase transformation from ilmenite to perovskite in FeTiO3 was directly observed using synchrotron-based X-ray diffraction and a large-volume press. The perovskite phase is temperature quenchable at 20 GPa and converts into the LiNbO3 phase at pressures below 15 GPa at room tem- perature. The LiNbO3 phase transforms into the ilmenite phase at 10 GPa and 673 K. However, the back-transformation from the ilmenite to the LiNbO3 phase was not observed, thus strongly suggesting that the LiNbO3 phase is not thermodynamically stable but rather a retrogressive phase formed from perovskite during decompression at room temperature. By cycling the pressure up and down at temperatures between 773 and 1023 K, the perovskite- ilmenite transformation could be observed in both directions, thus conÞ rming that perovskite is the true high-pressure phase with respect to the ilmenite phase at lower pressures. The phase boundary of the perovskite-ilmenite transformation thus determined in this study is represented by P (GPa) = 16.0 (±1.4) – 0.0012 (±0.0014) T (K), which is inconsistent with P = 25.2 – 0.01 T (K) reported previously (Syono et al. -

Anorogenic Alkaline Granites from Northeastern Brazil: Major, Trace, and Rare Earth Elements in Magmatic and Metamorphic Biotite and Na-Ma®C Mineralsq
Journal of Asian Earth Sciences 19 (2001) 375±397 www.elsevier.nl/locate/jseaes Anorogenic alkaline granites from northeastern Brazil: major, trace, and rare earth elements in magmatic and metamorphic biotite and Na-ma®c mineralsq J. Pla Cida,*, L.V.S. Nardia, H. ConceicËaÄob, B. Boninc aCurso de PoÂs-GraduacËaÄo em in GeocieÃncias UFRGS. Campus da Agronomia-Inst. de Geoc., Av. Bento GoncËalves, 9500, 91509-900 CEP RS Brazil bCPGG-PPPG/UFBA. Rua Caetano Moura, 123, Instituto de GeocieÃncias-UFBA, CEP- 40210-350, Salvador-BA Brazil cDepartement des Sciences de la Terre, Laboratoire de PeÂtrographie et Volcanologie-Universite Paris-Sud. Centre d'Orsay, Bat. 504, F-91504, Paris, France Accepted 29 August 2000 Abstract The anorogenic, alkaline silica-oversaturated Serra do Meio suite is located within the Riacho do Pontal fold belt, northeast Brazil. This suite, assumed to be Paleoproterozoic in age, encompasses metaluminous and peralkaline granites which have been deformed during the Neoproterozoic collisional event. Preserved late-magmatic to subsolidus amphiboles belong to the riebeckite±arfvedsonite and riebeckite± winchite solid solutions. Riebeckite±winchite is frequently rimmed by Ti±aegirine. Ti-aegirine cores are strongly enriched in Nb, Y, Hf, and REE, which signi®cantly decrease in concentrations towards the rims. REE patterns of Ti-aegirine are strikingly similar to Ti-pyroxenes from the IlõÂmaussaq peralkaline intrusion. Recrystallisation of mineral assemblages was associated with deformation although some original grains are still preserved. Magmatic annite was converted into magnetite and biotite with lower Fe/(Fe 1 Mg) ratios. Recrystallised amphibole is pure riebeckite. Magmatic Ti±Na-bearing pyroxene was converted to low-Ti aegirine 1 titanite ^ astrophyllite/aenigmatite. -
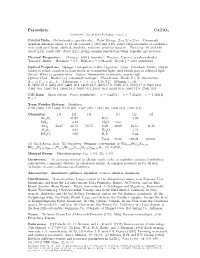
Perovskite Catio3 C 2001-2005 Mineral Data Publishing, Version 1 Crystal Data: Orthorhombic, Pseudocubic
Perovskite CaTiO3 c 2001-2005 Mineral Data Publishing, version 1 Crystal Data: Orthorhombic, pseudocubic. Point Group: 2/m 2/m 2/m. Commonly resemble distorted cubes, to 12 cm, striated k [001] and [110], rarely cubo-octahedra or octahedra, with additional forms, skeletal, dendritic; reniform, granular massive. Twinning: 90◦and 180◦ about [101], rarely 180◦ about [121], giving complex penetration twins; lamellar and sectored. Physical Properties: Cleavage: {001}, imperfect. Fracture: Uneven to subconchoidal. Tenacity: Brittle. Hardness = 5.5 D(meas.) = 3.98–4.26 D(calc.) = 4.02 (synthetic). Optical Properties: Opaque, transparent in thin fragments. Color: Iron-black, brown, reddish brown to yellow; colorless to dark brown in transmitted light; dark bluish gray in reflected light. Streak: White to grayish white. Luster: Adamantine to metallic; may be dull. Optical Class: Biaxial (+); commonly isotropic. Pleochroism: Weak; Z > X. Orientation: X = a; Y = c; Z = b. Dispersion: r> v. n= 2.34–2.37 2V(meas.) = 90◦ R: (400) 19.2, (420) 18.8, (440) 18.4, (460) 18.0, (480) 17.6, (500) 17.3, (520) 17.0, (540) 16.8, (560) 16.6, (580) 16.4, (600) 16.2, (620) 16.1, (640) 16.0, (660) 16.0, (680) 15.9, (700) 15.9 Cell Data: Space Group: P nma (synthetic). a = 5.447(1) b = 7.654(1) c = 5.388(1) Z=4 X-ray Powder Pattern: Synthetic. 2.701 (100), 1.911 (50), 2.719 (40), 1.557 (25), 1.563 (16), 3.824 (14), 1.567 (14) Chemistry: (1) (2) (3) (1) (2) (3) Nb2O5 25.99 FeO 5.69 SiO2 0.33 MgO trace TiO2 58.67 38.70 58.75 CaO 40.69 23.51 41.25 Al2O3 0.82 Na2O 1.72 RE2O3 3.08 K2O 0.44 Total 99.36 100.28 100.00 2+ (1) Val d’Aosta, Italy. -

Titanite Ores of the Khibiny Apatite-Nepheline- Deposits: Selective Mining, Processing and Application for Titanosilicate Synthesis
minerals Article Titanite Ores of the Khibiny Apatite-Nepheline- Deposits: Selective Mining, Processing and Application for Titanosilicate Synthesis Lidia G. Gerasimova 1,2, Anatoly I. Nikolaev 1,2,*, Marina V. Maslova 1,2, Ekaterina S. Shchukina 1,2, Gleb O. Samburov 2, Victor N. Yakovenchuk 1 and Gregory Yu. Ivanyuk 1 1 Nanomaterials Research Centre of Kola Science Centre, Russian Academy of Sciences, 14 Fersman Street, Apatity 184209, Russia; [email protected] (L.G.G.); [email protected] (M.V.M.); [email protected] (E.S.S.); [email protected] (V.N.Y.); [email protected] (G.Y.I.) 2 Tananaev Institute of Chemistry of Kola Science Centre, Russian Academy of Sciences, 26a Fersman Street, Apatity 184209, Russia; [email protected] * Correspondence: [email protected]; Tel.: +7-815-557-9231 Received: 4 September 2018; Accepted: 10 October 2018; Published: 12 October 2018 Abstract: Geological setting and mineral composition of (apatite)-nepheline-titanite ore from the Khibiny massif enable selective mining of titanite ore, and its processing with sulfuric-acid method, without preliminary concentration in flotation cells. In this process flow diagram, titanite losses are reduced by an order of magnitude in comparison with a conventional flotation technology. Further, dissolution of titanite in concentrated sulfuric acid produces titanyl sulfate, which, in turn, is a precursor for titanosilicate synthesis. In particular, synthetic analogues of the ivanyukite group minerals, SIV, was synthesized with hydrothermal method from the composition based on titanyl-sulfate, and assayed as a selective cation-exchanger for Cs and Sr. -

Electrocatalytic Properties of Calcium Titanate, Strontium Titanate, and Strontium Calcium Titanate Powders Synthesized by Solution Combustion Technique
Hindawi Advances in Materials Science and Engineering Volume 2019, Article ID 1612456, 7 pages https://doi.org/10.1155/2019/1612456 Research Article Electrocatalytic Properties of Calcium Titanate, Strontium Titanate, and Strontium Calcium Titanate Powders Synthesized by Solution Combustion Technique Oratai Jongprateep ,1,2 Nicha Sato ,1 Ratchatee Techapiesancharoenkij,1,2 and Krissada Surawathanawises1 1Department of Materials Engineering, Faculty of Engineering, Kasetsart University, Bangkok 10900, !ailand 2Materials Innovation Center, Faculty of Engineering, Kasetsart University, Bangkok 10900, !ailand Correspondence should be addressed to Oratai Jongprateep; [email protected] Received 29 October 2018; Accepted 13 February 2019; Published 4 April 2019 Academic Editor: Alexander Kromka Copyright © 2019 Oratai Jongprateep et al. *is is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Calcium titanate (CaTiO3), strontium titanate (SrTiO3), and strontium calcium titanate (SrxCa1−xTiO3) are widely recognized and utilized as dielectric materials. *eir electrocatalytic properties, however, have not been extensively examined. *e aim of this research is to explore the electrocatalytic performance of calcium titanate, strontium titanate, and strontium calcium titanate, as potential sensing materials. Experimental results revealed that CaTiO3, SrTiO3, and Sr0.5Ca0.5TiO3 powders synthesized by the solution combustion technique consisted of submicrometer-sized particles with 2 specific surface areas ranging from 4.19 to 5.98 m /g. Optical bandgap results indicated that while CaTiO3 and SrTiO3 had bandgap energies close to 3 eV, Sr0.5Ca0.5TiO3 yielded a lower bandgap energy of 2.6 eV. Cyclic voltammetry tests, measured in 0.1 M sodium nitrite, showed oxidation peaks occurring at 0.58 V applied voltage. -

Carbonation of Borehole Seals: Comparing Evidence from Short-Term Laboratory Experiments and Long-Term Natural Analogues Christopher A
Carbonation of borehole seals: Comparing evidence from short-term laboratory experiments and long-term natural analogues Christopher A. Rochelle* and Antoni E. Milodowski British Geological Survey, Environmental Science Centre, Nicker Hill, Keyworth, Nottingham, NG12 5GG, UK * Corresponding author: [email protected], tel +44 115 9363259, fax +44 115 363200 Abstract It is crucial that the engineered seals of boreholes in the vicinity of a deep storage facility remain effective for considerable timescales if the long-term geological containment of stored CO2 is to be effective. These timescales extend beyond those achievable by laboratory experiments or industrial experience. Study of the carbonation of natural Ca silicate hydrate (CSH) phases provides a useful insight into the alteration processes and evolution of cement phases over long-timescales more comparable with those considered in performance assessments. Samples from two such natural analogues in Northern Ireland have been compared with samples from laboratory experiments on the carbonation of Portland cement. Samples showed similar carbonation reaction processes even though the natural and experimental samples underwent carbonation under very different conditions and timescales. These included conversion of the CSH phases to CaCO3 and SiO2, and the formation of a well-defined reaction front. In laboratory experiments the reaction front is associated with localised Ca migration, localised matrix porosity increase, and localised shrinkage of the cement matrix with concomitant cracking. Behind the reaction front is a zone of CaCO3 precipitation that partly seals porosity. A broader and more porous/permeable reaction zone was created in the laboratory experiments compared to the natural samples, and it is possible that short-term experiments might not fully replicate slower, longer-term processes. -

Wang Et Al., 2001
American Mineralogist, Volume 86, pages 790–806, 2001 Characterization and comparison of structural and compositional features of planetary quadrilateral pyroxenes by Raman spectroscopy ALIAN WANG,* BRAD L. JOLLIFF, LARRY A. HASKIN, KARLA E. KUEBLER, AND KAREN M. VISKUPIC Department of Earth and Planetary Sciences and McDonnell Center for the Space Sciences, Washington University, St. Louis, Missouri 63130, U.S.A. ABSTRACT This study reports the use of Raman spectral features to characterize the structural and composi- tional characteristics of different types of pyroxene from rocks as might be carried out using a por- table field spectrometer or by planetary on-surface exploration. Samples studied include lunar rocks, martian meteorites, and terrestrial rocks. The major structural types of quadrilateral pyroxene can be identified using their Raman spectral pattern and peak positions. Values of Mg/(Mg + Fe + Ca) of pyroxene in the (Mg, Fe, Ca) quadrilateral can be determined within an accuracy of ±0.1. The preci- sion for Ca/(Mg + Fe + Ca) values derived from Raman data is about the same, except that correc- tions must be made for very low-Ca and very high-Ca samples. Pyroxenes from basalts can be distinguished from those in plutonic equivalents from the distribution of their Mg′ [Mg/(Mg + Fe)] and Wo values, and this can be readily done using point-counting Raman measurements on unpre- pared rock samples. The correlation of Raman peak positions and spectral pattern provides criteria to distinguish pyroxenes with high proportions of non-quadrilateral components from (Mg, Fe, Ca) quadrilateral pyroxenes. INTRODUCTION pyroxene group of minerals is amenable to such identification Laser Raman spectroscopy is well suited for characteriza- and characterization. -

BREDIGITE, LARNITE and ? DICALCIUM SILICATES from MARBLE CANYON Tnolras E
THE AMERICAN MINERALOGIST, VOL. 51, NOVEMBER_DECEMBER, 1966 BREDIGITE, LARNITE AND ? DICALCIUM SILICATES FROM MARBLE CANYON Tnolras E. Bnrocn, DepartmentoJ Geosciences,Texas T echnological College, Lubbo ck, T enas.r Alstnact The a' (bredigite), p (larnite) and 7 (unnamed) forms of dicalcium silicate (CarSiOr) occur together in the contact zone around a syenite-monzonite intrusion in Marble Canyon. Bredigite, larnite and 7 have consistant crystallographic orientations when they occur together. These relationships reflect the crystallographic direction in which the reorganiza- tion occurs when transformation from one form to another takes place. INrnooucrroN Two polymorphic forms of dicalcium silicate (CazSiOD have been de- scribed as naturally occurring minerals by Tilley (1929) and Tilley and Vincent (1948).The mineralswere found in a contact zonein Larne, Ire- Iand and given the names larnite and bredigite. Until 1964,no natural occurrencesof theseminerals had been reported from any locality in the United States. In 1963, three polymorphic forms of CazSiOawere found in the contact zone around a syenite-monzonite intrusion in 1\4arbleCavon. LocarroN AND SETTTNG Marble Canyon is in the east rim of the Diablo Plateau in Culberson County, Trans-PecosTexas, 30 miles north of the town of Van Horn and 2 miles west of State Highway 54. The canyon is about a mile long and terminates upstream in an elongate amphitheater roughly a mile and a half Iong and half a mile wide. In the center of this elongate amphitheater is an elliptical outcrop of five different types of igneous rock that are arranged in a concentric pattern. From the center out they are: (1) a coarse-grained syenite; (2) a coarse-grained green monzonitel (3) a medium-grained gray monzonite; (4) a discontinuous narrow border of olivine gabbro; and (5) small rhyolite dikes which cut the other rock types.