Acute Effects of Angiotensin II Receptor Blocker on Ventricular Repolarization Alternans in Chronic Heart Failure
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Young Adults. Look for ST Elevation, Tall QRS Voltage, "Fishhook" Deformity at the J Point, and Prominent T Waves
EKG Abnormalities I. Early repolarization abnormality: A. A normal variant. Early repolarization is most often seen in healthy young adults. Look for ST elevation, tall QRS voltage, "fishhook" deformity at the J point, and prominent T waves. ST segment elevation is maximal in leads with tallest R waves. Note high take off of the ST segment in leads V4-6; the ST elevation in V2-3 is generally seen in most normal ECG's; the ST elevation in V2- 6 is concave upwards, another characteristic of this normal variant. Characteristics’ of early repolarization • notching or slurring of the terminal portion of the QRS wave • symmetric concordant T waves of large amplitude • relative temporal stability • most commonly presents in the precordial leads but often associated with it is less pronounced ST segment elevation in the limb leads To differentiate from anterior MI • the initial part of the ST segment is usually flat or convex upward in AMI • reciprocal ST depression may be present in AMI but not in early repolarization • ST segments in early repolarization are usually <2 mm (but have been reported up to 4 mm) To differentiate from pericarditis • the ST changes are more widespread in pericarditis • the T wave is normal in pericarditis • the ratio of the degree of ST elevation (measured using the PR segment as the baseline) to the height of the T wave is greater than 0.25 in V6 in pericarditis. 1 II. Acute Pericarditis: Stage 1 Pericarditis Changes A. Timing 1. Onset: Day 2-3 2. Duration: Up to 2 weeks B. Findings 1. -

Interpreting CVP Waveforms Summary EK
INTERPRETING CENTRAL VENOUS PRESSURE WAVEFORMS IN TERPRETING CEN TRAL VEN OUS PRESSURE W AVEFORMS INTRODUCTION A central venous pressure waveform provides important information about the hemodynamic state of the patient and compliance of the syste m. - CENTRAL VEN OUS PRESSURE (CVP) W AVEFORMS • Measures pressure in the central venous circulation • Obtained by using a central venous catheter ideally placed at the junction of the superior vena cava (SVC) and the right atrium • Depends on: 1. Compliance of the cardiovascular system 2. Overall volume status Figure 1: Central venou s catheter placement at the SV C-RA junction INTERPRETING CVP W AVEFORMS Remember that the waveform represents atrial pressure which is the relationship between atrial compliance and volume. One CVP Waveform represents one complete beat of the heart. • a-wave: atrial contraction • Corresponds with timing of P wave on ECG • Noncompliant right atrium: = large a-wave • c-wave: movement of the tric uspid valve into the right atrium • Corresponds with timing of R wave on ECG • Transient decrease in atrial compliance/increase in atrial pressure • c-wave and a-wave can be difficult to differentiate when patient is ta tachycardic • x-descent: atrial relaxation • Corresponds with timing of QRS complex on ECG Figure 2: The different parts of a central venous • Tricuspid valve descends towards the apex of the right ventricle pressure waveform causing a decrease in right atrial pressure • v-wave: atrial filling • Corresponds with timing of T wave on ECG • A large v-wave may indicate tricuspid regurgitation QRS • If right atrium is very compliant, tricuspid regurgitation is possible complex without generation of large v-wave • y-descent: atrial emptying • Corresponds with timing just prior to P wave on ECG P wave T wave Figure 3: ECG showing timing of CVP waveform components This document is meant to be used as an educational resource for physicians and other healthcare professionals. -

Ventricular Repolarization Components on the Electrocardiogram Cellular Basis and Clinical Significance Gan-Xin Yan, MD, PHD, Ramarao S
View metadata, citation and similar papers at core.ac.uk brought to you by CORE Journal of the American College of Cardiology providedVol. by Elsevier 42, No. - 3,Publisher 2003 Connector © 2003 by the American College of Cardiology Foundation ISSN 0735-1097/03/$30.00 Published by Elsevier Inc. doi:10.1016/S0735-1097(03)00713-7 STATE-OF-THE-ART PAPER Ventricular Repolarization Components on the Electrocardiogram Cellular Basis and Clinical Significance Gan-Xin Yan, MD, PHD, Ramarao S. Lankipalli, MD, James F. Burke, MD, FACC, Simone Musco, MD, Peter R. Kowey, MD, FACC Wynnewood, Pennsylvania Ventricular repolarization components on the surface electrocardiogram (ECG) include J (Osborn) waves, ST-segments, and T- and U-waves, which dynamically change in morphol- ogy under various pathophysiologic conditions and play an important role in the development of ventricular arrhythmias. Our primary objective in this review is to identify the ionic and cellular basis for ventricular repolarization components on the body surface ECG under normal and pathologic conditions, including a discussion of their clinical significance. A specific attempt to combine typical clinical ECG tracings with transmembrane electrical recordings is made to illustrate their logical linkage. A transmural voltage gradient during initial ventricular repolarization, which results from the presence of a prominent transient ϩ outward K current (Ito)-mediated action potential (AP) notch in the epicardium, but not endocardium, manifests as a J-wave on the ECG. The J-wave is associated with the early repolarization syndrome and Brugada syndrome. ST-segment elevation, as seen in Brugada syndrome and acute myocardial ischemia, cannot be fully explained by using the classic concept of an “injury current” that flows from injured to uninjured myocardium. -

The Heart the Pulse and the ECG Booklet.Indd
AF A ® www.afa.org.uk The heart, the pulse and the electrocardiograph Providing information, support and access to established, new or innovative treatments for atrial fi brillation www.afa.org.uk Registered Charity No. 1122442 Glossary Arrhythmia Heart rhythm disorder Contents Arrhythmia Nurse Specialist A nurse who is Glossary trained in heart rhythm disorders The heart Atrial Fibrillation (AF) An irregular heart rhythm due to a rapid, disorganised electrical disturbance of The pulse the heart’s upper chambers (the atria) How to take a pulse Atrial Flutter (Afl ) A rhythm disorder of a more organised electrical disturbance in the heart’s upper The electrocardiograph chambers. The heart rhythm may be either regular (ECG) or irregular Bradycardia A rhythm disorder characterised by a slow heart rate of less than 60 beats per minute Cardiologist A doctor who specialises in the diagnosis and treatment of patients with heart conditions Echocardiogram An image of the heart using echocardiography or soundwave-based technology. An echocardiogram (echo) shows a three dimensional shot of the heart Electrocardiograph (ECG) A 2D graphic of the heart’s electrical activity. An ECG is taken from electrodes on the skin surface Heart Failure The inability (failure) of the heart to pump suffi cient oxygenated blood around the body to meet physiological requirements Sinus Rhythm Normal behaviour of the heart Syncope Fainting/passing out from a temporary lack of oxygen going to certain areas of the brain Tachycardia A rhythm disorder characterised by a rapid heart rate of more than 100 beats per minute 2 The heart The heart Structurally, the heart consists of two sides, a right and normal and a left. -

Basic Cardiac Rhythms – Identification and Response Module 1 ANATOMY, PHYSIOLOGY, & ELECTRICAL CONDUCTION Objectives
Basic Cardiac Rhythms – Identification and Response Module 1 ANATOMY, PHYSIOLOGY, & ELECTRICAL CONDUCTION Objectives ▪ Describe the normal cardiac anatomy and physiology and normal electrical conduction through the heart. ▪ Identify and relate waveforms to the cardiac cycle. Cardiac Anatomy ▪ 2 upper chambers ▪ Right and left atria ▪ 2 lower chambers ▪ Right and left ventricle ▪ 2 Atrioventricular valves (Mitral & Tricuspid) ▪ Open with ventricular diastole ▪ Close with ventricular systole ▪ 2 Semilunar Valves (Aortic & Pulmonic) ▪ Open with ventricular systole ▪ Open with ventricular diastole The Cardiovascular System ▪ Pulmonary Circulation ▪ Unoxygenated – right side of the heart ▪ Systemic Circulation ▪ Oxygenated – left side of the heart Anatomy Coronary Arteries How The Heart Works Anatomy Coronary Arteries ▪ 2 major vessels of the coronary circulation ▪ Left main coronary artery ▪ Left anterior descending and circumflex branches ▪ Right main coronary artery ▪ The left and right coronary arteries originate at the base of the aorta from openings called the coronary ostia behind the aortic valve leaflets. Physiology Blood Flow Unoxygenated blood flows from inferior and superior vena cava Right Atrium Tricuspid Valve Right Ventricle Pulmonic Valve Lungs Through Pulmonary system Physiology Blood Flow Oxygenated blood flows from the pulmonary veins Left Atrium Mitral Valve Left Ventricle Aortic Valve Systemic Circulation ▪ Blood Flow Through The Heart ▪ Cardiology Rap Physiology ▪ Cardiac cycle ▪ Represents the actual time sequence between -
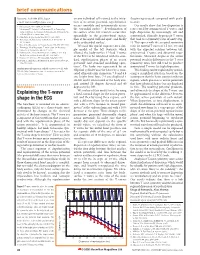
Explaining the T-Wave Shape in The
brief communications Inuyama, Aichi 484-8506, Japan on any individual cell is timed to the initia- (beginning-to-peak compared with peak- e-mail: [email protected] tion of its action potential, repolarization to-end). 1. Matsuzawa, T. Nature 315, 57–59 (1985). occurs smoothly and systematically across Our results show that low dispersion is 5,6 2. Matsuzawa, T., Itakura, S. & Tomonaga, M. in Primatology the epicardial surface . Repolarization of represented by asymmetrical T waves, and Today (eds Ehara, A., Kumura, T., Takenaka, O. & Iwamoto, M.) the surface of the left ventricle occurs first high dispersion by increasingly tall and 317–320 (Elsevier, Amsterdam, 1991). epicardially in the postero-basal region, symmetrical, clinically hyperacute T waves 3. Murofushi, K. Jpn. Psychol. Res. 39, 140–153 (1997). 6 4. Tomonaga, M., Matsuzawa, T. & Itakura, S. Primate Res. 9, then at the septal wall and apex , and finally that tend to a symmetry ratio of unity (Fig. 67–77 (1993). at the endocardial surface7. 1). This agrees with the accepted symmetry 5. Biro, D. & Matsuzawa, T. J. Comp. Psychol. 113, 178–185 (1999). We used this spatial sequence on a sim- ratio for normal T waves of 1.5 (ref. 10) and 6. Tomonaga, M. & Matsuzawa, T. Anim. Cogn. (in the press). 7. Miller, G. A. Psychol. Rev. 63, 81–97 (1956). ple model of the left ventricle which with the expected relation between tall, 8. Rumbaugh, D., Savage-Rumbaugh, E. S. & Hegel, M. J. Exp. allowed the body-surface 12-lead T waves symmetrical T waves and abnormal repo- Psychol. -

Effect of Intravenous Adrenaline on Electrocardiogram, Blood Pressure, and Serum Potassium
Br Heart J: first published as 10.1136/hrt.49.1.90 on 1 January 1983. Downloaded from Br Heart J 1983; 49: 90-3 Effect of intravenous adrenaline on electrocardiogram, blood pressure, and serum potassium A D STRUTHERS, J L REID, R WHITESMITH, J C RODGER From the University Department ofMateria Medica, and Department ofBiochemistry, StobhiU General Hospital, Glasgow, and Medical Unit, Monklands and District General Hospital, Airdrie, Lanarkshire SUMMARY Increased catecholamines after myocardial infarction may contribute to the development ofarrhythmias. We have infused adrenaline intravenously in nine normal volunteers to levels similar to those seen after myocardial infarction. Adrenaline caused an increase in systolic blood pressure, a decrease in diastolic blood pressure, and an increase in heart rate. Adrenaline also produced a decrease in T wave amplitude and an increase in the QTc interval. The serum potassium fell dramatically during the adrenaline infusion from a control value of 4*06 mmot/l to 3-22 mmol/l. Hypokalaemia after myocardial infarction is associated with an increased incidence of ventricular arrhythmias. Thus, circulating adrenaline may increase the frequency of arrhythmias both directly via changes in ventricular repolarisation and indirectly via adrenaline induced hypokalaemia. Hypokalaemia is not uncommonly observed in the All subjects gave their informed consent to the acute phase of myocardial infarctionl 2 when it is investigation which had the approval of the hospital's associated with serious ventricular arrhythmias.3 We research and ethical committee. have found that in this circumstance hypokalaemia is Subjects were investigated in the morning after a http://heart.bmj.com/ transient, it resolves without potassium supplements, standard light breakfast. -

Dysrhythmias
CARDIOVASCULAR DISORDERS DYSRHYTHMIAS I. BASIC PRINCIPLES OF CARDIAC CONDUCTION DISTURBANCES A. Standard ECG and rhythm strips 1. Recordings are obtained at a paper speed of 25 mm/sec. 2. The vertical axis measures distance; the smallest divisions are 1 mm ×1 mm. 3. The horizontal axis measures time; each small division is 0.04 sec/mm. B. Normal morphology Courtesy of Dr. Michael McCrea 1. P wave = atrial depolarization a. Upright in leads I, II, III, aVL, and aVF; inverted in lead aVR b. Measures <0.10 seconds wide and <3 mm high c. Normal PR interval is 0.12–0.20 seconds. 2. QRS complex = ventricular depolarization a. Measures 0.06-0.10 seconds wide b. Q wave (1) <0.04 seconds wide and <3 mm deep (2) Abnormal if it is >3 mm deep or >1/3 of the QRS complex. c. R wave ≤7.5 mm high 3. QT interval varies with rate and sex but is usually 0.33–0.42 seconds; at normal heart rates, it is normally <1/2 the preceding RR interval. 4. T wave = ventricular repolarization a. Upright in leads I, II, V3–V6; inverted in aVR b. Slightly rounded and asymmetric in configuration c. Measures ≤5 mm high in limb leads and ≤10 mm high in the chest leads 5. U wave = a ventricular afterpotential a. Any deflection after the T wave (usually low voltage) b. Same polarity as the T wave c. Most easily detected in lead V3 d. Can be a normal component of the ECG e. Prominent U waves may indicate one of the following: (1) Hypokalemia (<3 mEq/L) (2) Hypercalcemia (3) Therapy with digitalis, phenothiazines, quinidine, epinephrine, inotropic agents, or amiodarone (4) Thyrotoxicosis f. -

The Enigmatic Sixth Wave of the Electrocardiogram: the U Wave
Cardiology Journal 2008, Vol. 15, No. 5, pp. 408–421 Copyright © 2008 Via Medica REVIEW ARTICLE ISSN 1897–5593 The enigmatic sixth wave of the electrocardiogram: The U wave Andrés Ricardo Pérez Riera1, Celso Ferreira1, Celso Ferreira Filho1, Marcelo Ferreira1, Adriano Meneghini1, Augusto Hiroshi Uchida2, Edgardo Schapachnik3, Sergio Dubner4, Li Zhang5 1For International VCG Investigators, ABC Faculty of Medicine (FMABC), Foundation of ABC (FUABC), Santo André, Brazil 2Heart Institute of the University of São Paulo Medical School, São Paulo, Brazil 3Dr. Cosme Argerich Hospital, Buenos Aires, Argentina 4Clínica and Maternidad Suizo Argentina, Buenos Aires, Argentina 5Main Line Health Heart Center, Jefferson Medical College, Philadelphia, PA, USA Electro-Vectorcardigraphic Section, ABC Medical School, ABC Foundation, Santo André, São Paulo, Brazil Abstract The U wave is the last, inconstant, smallest, rounded and upward deflection of the electrocar- diogram. Controversial in origin, it is sometimes seen following the T wave with the TU junction along the baseline or fused with it and before P of the following cycle on the TP segment. In this review we will study its temporal location related to monophasic action potential, cardiac cycle and heart sounds, polarity, voltage or amplitude, frequency and shape- contour. We will analyze the clinical significance of negative, alternant, prominent U wave, and the difference between T wave with two peaks (T1–T2) and true U wave. Finally we will analyze the four main hypotheses about the source of U wave: repolarization of the intraven- tricular conducting system or Purkinje fibers system, delayed repolarization of the papillary muscles, afterpotentials caused by mechanoelectrical hypothesis or mechanoelectrical feedback, and the prolonged repolarization in the cells of the mid-myocardium (“M-cells”). -

The U Wave in the Electrocardiogram. More Than an Academic Curiosity
ARTÍCULO DE REVISIÓN The U wave in the electrocardiogram. More than an academic curiosity Raúl Carrillo-Esper,* Luis Daniel Carrillo-Córdova,** Dulce María Carrillo-Córdova,*** Carlos Alberto Carrillo-Córdova*** RESUMENS ABSTRACTTC La onda U fue descrita por Einthoven en 1903. A pesar del tiempo The electrocardiographic U wave was describe by Einthoven in transcurrido desde su descripción inicial las bases electrofisiológi- 1903. Despite the time elapsed since its description the electrophy- cas que expliquen su génesis no son claras, por lo que se han siological basis underlying the genesis of the U wave has not been propuesto varias teorías. La adecuada evaluación e interpretación precisely elucidated and several hypothesis have been proposed. de la onda U es importante en la práctica clínica, ya que su presen- Proper evaluation and interpretation of the U wave is useful to the cia puede ser marcador de enfermedad cardiaca. El objetivo de clinician because it is a marker of heart disease. The aim of this este artículo es revisar los conceptos actuales relacionados con la paper is to review current concepts related to U wave, its genesis, onda U, su génesis, significado y adecuada interpretación. significance and proper interpretation. Palabrasb s clave.v . Onda U. Electrocardiograma. Cardiopatía is- Keyy words.d dKey U wave. Electrocardiogram. Ischemic heart disease. quémica. THE U WAVE IN THE ELECTROCARDIGRAM. MORE THAN ACADEMIC CURIOSITY Willem Einthoven developed the first electrocardiogra- ph machine. It was a simple string galvanometer capable of mesuring small changes in the electrical potential as the heart contracted and relaxed (Figure 1). After having decribed the electrocardiogram (ECG) in 1895, Einthoven first recognized in 1903 the U wave and extended the notion of the ECG in a seminal paper publis- hed in the Lancet in 1912. -

Advanced EKG Interpretation JUNCTIONAL RHYTHMS and NURSING INTERVENTIONS Objectives
Advanced EKG Interpretation JUNCTIONAL RHYTHMS AND NURSING INTERVENTIONS Objectives ♥ Identify specific cardiac dysrhythmias ♥ Describe appropriate nursing interventions for specific dysrhythmias Junctional Rhythms ▪ Junctional rhythms are named such because their impulse originates from the AV node (AV junction) instead of the SA node. ▪ The SA node may be impaired secondary to drug toxicity or underlying cardiac disease. ▪ When the AV node does not sense an impulse coming down from the SA node, it will become the pacemaker of the heart. Characteristics of all Junctional Rhythms ▪ Inverted (negative) or absent P waves are seen before each QRS complex OR ▪ P wave can be hidden in the QRS complex OR ▪ P wave may follow the QRS complex ▪ PR interval of <0.12 seconds (remember normal is 0.12-0.2) ▪ QRS complex within normal measurements Most Common Variations ▪ Junctional (escape) rhythm: 40 - 60 bpm ▪ Accelerated junctional rhythm: 61 – 100 bpm ▪ Junctional tachycardia: >100 bpm ▪ Premature junctional complexes (PJCs) Junctional Rhythm ♥ Junctional (escape) rhythms originate at or around the AV node and the Bundle of His. The impulse travels up the atria and down to the ventricles resulting in inverted P waves that can occur prior to, during or after the QRS. ♥ P waves can also be absent if the impulse does not travel up into the atria. Inverted P wave 5 Steps to Identify Junctional Rhythm 1. What is the rate? 40-60 bpm 2. What is the rhythm? Regular 3. Is there a P wave before each QRS? Are P waves upright Usually inverted or absent, may be before, during or after and uniform? QRS complex 4. -
Dr. Thouraya Said Objectives
Dr. Thouraya Said Objectives Identify and understand: ü The events causing the different waves of the JVP & CAP tracings. ü Difference between JVP and CAP. ü Correlation between JVP, CAP, ECG and Phoncardiogram. The carotid pulse tells about the aorta and left ventricular function. JVP provides information regarding hemodynamic changes in the right side of the heart. Evaluation of pulse waveform helps in the diagnosis of certain cardiac diseases & assessing their severity. Distinguishing features between venous and arterial pulses JVP CAP Visible but not palpable Palpable Obliterated by pressure Not obliterated by pressure 2 pulsations per systole 1 pulsation per systole Decreases with inspiration No effect of respiration Enhanced by H-J-Reflex No effect of abdominal pressure Carotid arterial pressure q The carotid pulse can be taken on the right side of the neck over the carotid artery in order to determine heart rate. q When blood is forced into the aorta during ventricular systole, two things happen: 1. Blood is moved forwards. 2. A pressure wave is set up which travels along the wall of arteries (faster than the flow of blood), expanding the arterial walls as it travels. The expansion of the arterial wall is palpable as the pulse. How to examine 1. Subject supine at 30° head slightly bent to the examined side. 2. Feel CAP on medial side of SCM alongside the lateral border of thyroid cartilage. 3. Apply transducer over CAP using soft rubber band & connect it to recorder. Recorded CAP graph q Anacrotic limb (rapid upstroke): Sharp rise in pressure to a peak of 120mmHg during maximum ejection phase of ventricular systole.