Deep Phylogeny, Ancestral Groups and the Four Ages of Life
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Activity-Release-Form-KLCC
Kingdom Life Community Church Activity Release Form For those 18 years of age or older, all parents, and all guardians: I consent for any of my children listed below to participate in any activity or trip sponsored by Kingdom Life Community Church. In case of medical need or injury, I authorize the church to arrange for medical or dental services for me and any of my children listed below. I agree that any such expense will be my obligation. I, individually, or in my capacities as parent or guardian of the below listed children, waive, release, and indemnify the church and all of its agents, directors, officers, employees and volunteers (collectively, “released parties”) from all demands, claims, liability, in law or in equity, that have arisen or may arise from any church activity or trip and that involve any damage, loss, or injury to me, my spouse, any of my children, my property, or the property of any of my children. In the same capacities, I promise not to sue any of the released parties for any such demands, claims, or liability. This waiver, release, indemnification, and promise not to sue do not apply to claims of criminal conduct or gross negligence. I understand that the church may take photographs of me and my family in the course of its activities, and I grant the church permission to publish such photographs in a manner the church deems appropriate. This activity release form is in effect for any activities that I, or any of my children, may participate in. This form is revocable, prospectively only, by a writing signed by me that bears the date that the revocation is delivered to the church. -

Buzzle – Zoology Terms – Glossary of Biology Terms and Definitions Http
Buzzle – Zoology Terms – Glossary of Biology Terms and Definitions http://www.buzzle.com/articles/biology-terms-glossary-of-biology-terms-and- definitions.html#ZoologyGlossary Biology is the branch of science concerned with the study of life: structure, growth, functioning and evolution of living things. This discipline of science comprises three sub-disciplines that are botany (study of plants), Zoology (study of animals) and Microbiology (study of microorganisms). This vast subject of science involves the usage of myriads of biology terms, which are essential to be comprehended correctly. People involved in the science field encounter innumerable jargons during their study, research or work. Moreover, since science is a part of everybody's life, it is something that is important to all individuals. A Abdomen: Abdomen in mammals is the portion of the body which is located below the rib cage, and in arthropods below the thorax. It is the cavity that contains stomach, intestines, etc. Abscission: Abscission is a process of shedding or separating part of an organism from the rest of it. Common examples are that of, plant parts like leaves, fruits, flowers and bark being separated from the plant. Accidental: Accidental refers to the occurrences or existence of all those species that would not be found in a particular region under normal circumstances. Acclimation: Acclimation refers to the morphological and/or physiological changes experienced by various organisms to adapt or accustom themselves to a new climate or environment. Active Transport: The movement of cellular substances like ions or molecules by traveling across the membrane, towards a higher level of concentration while consuming energy. -

Revised Glossary for AQA GCSE Biology Student Book
Biology Glossary amino acids small molecules from which proteins are A built abiotic factor physical or non-living conditions amylase a digestive enzyme (carbohydrase) that that affect the distribution of a population in an breaks down starch ecosystem, such as light, temperature, soil pH anaerobic respiration respiration without using absorption the process by which soluble products oxygen of digestion move into the blood from the small intestine antibacterial chemicals chemicals produced by plants as a defence mechanism; the amount abstinence method of contraception whereby the produced will increase if the plant is under attack couple refrains from intercourse, particularly when an egg might be in the oviduct antibiotic e.g. penicillin; medicines that work inside the body to kill bacterial pathogens accommodation ability of the eyes to change focus antibody protein normally present in the body acid rain rain water which is made more acidic by or produced in response to an antigen, which it pollutant gases neutralises, thus producing an immune response active site the place on an enzyme where the antimicrobial resistance (AMR) an increasing substrate molecule binds problem in the twenty-first century whereby active transport in active transport, cells use energy bacteria have evolved to develop resistance against to transport substances through cell membranes antibiotics due to their overuse against a concentration gradient antiretroviral drugs drugs used to treat HIV adaptation features that organisms have to help infections; they -

THE CASE AGAINST Marine Mammals in Captivity Authors: Naomi A
s l a m m a y t T i M S N v I i A e G t A n i p E S r a A C a C E H n T M i THE CASE AGAINST Marine Mammals in Captivity The Humane Society of the United State s/ World Society for the Protection of Animals 2009 1 1 1 2 0 A M , n o t s o g B r o . 1 a 0 s 2 u - e a t i p s u S w , t e e r t S h t u o S 9 8 THE CASE AGAINST Marine Mammals in Captivity Authors: Naomi A. Rose, E.C.M. Parsons, and Richard Farinato, 4th edition Editors: Naomi A. Rose and Debra Firmani, 4th edition ©2009 The Humane Society of the United States and the World Society for the Protection of Animals. All rights reserved. ©2008 The HSUS. All rights reserved. Printed on recycled paper, acid free and elemental chlorine free, with soy-based ink. Cover: ©iStockphoto.com/Ying Ying Wong Overview n the debate over marine mammals in captivity, the of the natural environment. The truth is that marine mammals have evolved physically and behaviorally to survive these rigors. public display industry maintains that marine mammal For example, nearly every kind of marine mammal, from sea lion Iexhibits serve a valuable conservation function, people to dolphin, travels large distances daily in a search for food. In learn important information from seeing live animals, and captivity, natural feeding and foraging patterns are completely lost. -

The Phagotrophic Origin of Eukaryotes and Phylogenetic Classification Of
International Journal of Systematic and Evolutionary Microbiology (2002), 52, 297–354 DOI: 10.1099/ijs.0.02058-0 The phagotrophic origin of eukaryotes and phylogenetic classification of Protozoa Department of Zoology, T. Cavalier-Smith University of Oxford, South Parks Road, Oxford OX1 3PS, UK Tel: j44 1865 281065. Fax: j44 1865 281310. e-mail: tom.cavalier-smith!zoo.ox.ac.uk Eukaryotes and archaebacteria form the clade neomura and are sisters, as shown decisively by genes fragmented only in archaebacteria and by many sequence trees. This sisterhood refutes all theories that eukaryotes originated by merging an archaebacterium and an α-proteobacterium, which also fail to account for numerous features shared specifically by eukaryotes and actinobacteria. I revise the phagotrophy theory of eukaryote origins by arguing that the essentially autogenous origins of most eukaryotic cell properties (phagotrophy, endomembrane system including peroxisomes, cytoskeleton, nucleus, mitosis and sex) partially overlapped and were synergistic with the symbiogenetic origin of mitochondria from an α-proteobacterium. These radical innovations occurred in a derivative of the neomuran common ancestor, which itself had evolved immediately prior to the divergence of eukaryotes and archaebacteria by drastic alterations to its eubacterial ancestor, an actinobacterial posibacterium able to make sterols, by replacing murein peptidoglycan by N-linked glycoproteins and a multitude of other shared neomuran novelties. The conversion of the rigid neomuran wall into a flexible surface coat and the associated origin of phagotrophy were instrumental in the evolution of the endomembrane system, cytoskeleton, nuclear organization and division and sexual life-cycles. Cilia evolved not by symbiogenesis but by autogenous specialization of the cytoskeleton. -
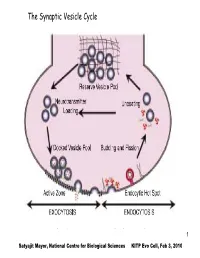
The Synaptic Vesicle Cycle
The Synaptic Vesicle Cycle 1 Satyajit Mayor, National Centre for Biological Sciences KITP Evo Cell, Feb 3, 2010 The Eukaryotic Commandments: T. Cavalier-Smith (i)origin of the endomembrane system (ER, Golgi and lysosomes) and coated- vesicle budding and fusion, including endocytosis and exocytosis; (ii) origin of the cytoskeleton, centrioles, cilia and associated molecular motors; (iii) origin of the nucleus, nuclear pore complex and trans-envelope protein and RNA transport; (iv) origin of linear chromosomes with plural replicons, centromeres and telomeres; (v) origin of novel cell-cycle controls and mitotic segregation; (vi) origin of sex (syngamy, nuclear fusion and meiosis); (vii) origin of peroxisomes; (ix) origin of mitochondria; (viii) novel patterns of rRNAprocessing using small nucleolar-ribonucleoproteins (snoRNPs); (x) origin of spliceosomal introns. 2 EM of Chemical synapse mitochondria Active Zone Figure 5.3, Bear, 2001 3 T. Cavalier-Smith International Journal of Systematic and Evolutionary Microbiology (2002), 52, 297–354 Fig. 1. The bacterial origins of eukaryotes as a two-stage process. I phase: The ancestors of eukaryotes, the stem neomura, are shared with archaebacteria and evolved during the neomuran revolution, in which N-linked glycoproteins replaced murein peptidoglycan and 18 other suites of characters changed radically through adaptation of an ancestral actinobacterium to thermophily, as discussed in detail by Cavalier- Smith (2002a). 4 T. Cavalier-Smith International Journal of Systematic and Evolutionary Microbiology (2002), 52, 297–354 Fig. 1. The bacterial origins of eukaryotes as a two-stage process. II phase: In the next phase, archaebacteria and eukaryotes diverged dramatically. Archaebacteria retained the wall and therefore their general bacterial cell and genetic organization, but became adapted to even hotter and more acidic environments by substituting prenyl ether lipids for the ancestral acyl esters and making new acid- resistant flagellar shafts (Cavalier-Smith, 2002a). -

Card Uses a Minor Groove Wedge Mechanism to Stabilize the RNA
1 CarD uses a minor groove wedge mechanism to stabilize the RNA 2 polymerase open promoter complex 3 4 Brian Bae1, James Chen1, Elizabeth Davis1, Katherine Leon1, Seth A. Darst1,*, 5 Elizabeth A. Campbell1,* 6 7 1The Rockefeller University, Laboratory for Molecular Biophysics, 1230 York Avenue, New York, 8 NY 10065, USA. 9 10 *Correspondence to: E-mail: [email protected], [email protected] 11 12 Present Address: Elizabeth Davis, The University of Minnesota School of Medicine, 13 420 Delaware St. SE, Minneapolis, MN 55455, USA; Katherine Leon, Department of 14 Biochemistry and Molecular Biology, University of Chicago, 929 East 57th Street, GCIS 15 W219 Chicago, IL 60637, USA. 16 17 2 18 Abstract A key point to regulate gene expression is at transcription initiation, and 19 activators play a major role. CarD, an essential activator in Mycobacterium tuberculosis, 20 is found in many bacteria, including Thermus species, but absent in Escherichia coli. To 21 delineate the molecular mechanism of CarD, we determined crystal structures of 22 Thermus transcription initiation complexes containing CarD. The structures show CarD 23 interacts with the unique DNA topology presented by the upstream double- 24 stranded/single-stranded DNA junction of the transcription bubble. We confirm that our 25 structures correspond to functional activation complexes, and extend our understanding 26 of the role of a conserved CarD Trp residue that serves as a minor groove wedge, 27 preventing collapse of the transcription bubble to stabilize the transcription initiation 28 complex. Unlike E. coli RNAP, many bacterial RNAPs form unstable promoter 29 complexes, explaining the need for CarD. -

Marine Microbiology at Scripps
81832_Ocean 8/28/03 7:18 PM Page 67 Special Issue—Scripps Centennial Marine Microbiology at Scripps A. Aristides Yayanos Scripps Institution of Oceanography, University of California, • San Diego, California USA Marine microbiology is the study of the smallest isolated marine bioluminescent bacteria, isolated and organisms found in the oceans—bacteria and archaea, characterized sulfate-reducing bacteria, and showed many eukaryotes (among the protozoa, fungi, and denitrifying bacteria could both produce and consume plants), and viruses. Most microorganisms can be seen nitrous oxide, now known to be an important green- only with a microscope. Microbes pervade the oceans, house gas. Beijerinck also founded the field of virology its sediments, and some hydrothermal fluids and through his work on plant viruses (van Iterson et al., exhibit solitary life styles as well as complex relation- 1983). Mills (1989) describes the significance of the ships with animals, other microorganisms, and each work of Beijerink and Winogradsky to plankton other. The skeletal remains of microorganisms form the research and marine chemistry. largest component of sedimentary fossils whose study Around 1903, bacteriology in California was reveals Earth’s history. The enormous morphological, emerging in the areas of medicine and public health physiological, and taxonomic diversity of marine and accordingly was developing into an academic dis- microorganisms remains far from adequately cipline in medical schools (McClung and Meyer, 1974). described and studied. Because the sea receives terres- Whereas the branch of microbiology dealing with bac- trial microorganisms from rivers, sewage outfalls, and teria and viruses was just beginning, the branch con- other sources, marine microbiology also includes the cerning protozoa and algae was a relatively more estab- study of alien microorganisms. -

Activity 3: Six Kingdoms Brochure
Activity 3: Six Kingdoms Brochure Objective: You will demonstrate your knowledge of the six kingdoms of organisms by gathering information (from your class notes, the internet, and the biology textook) and creating a brochure on the six kingdoms in which scientists classify organisms. Your brochure will be organized as follows: 1. Making the Brochure- the brochure is made of one piece of paper. Fold the paper into thirds. 2. Cover- your cover should have a picture and an appropriate title. Your name should be written in the bottom right corner of the cover. 3. Inside the Brochure- inside your brochure, you should have one section for each of six kingdoms. Use the front and back of the paper. Since there will only be five open sections left in the brochure, you should place both the Eubacteria Kingdom and Archaebacteria Kingdom in the same section. You must include the following information for each of the six kingdoms: • Are the organisms unicellular (one cell) or multicellular (many cells) or both? • Do they have a nucleus in their cells? • Do they make their own food or get it from other organisms? • Other important characteristics • A picture or a diagram of sample organisms (one or a few) The Kingdom Fungi The Kingdom Fungi includes some of the most important organisms, both in terms of their ecological and economic roles. By decomposing dead material, they continue the cycle of nutrients through ecosystems. In addition, most plants could not grow without the fungi, or mycorrhizae, that live in their roots and supply essential nutrients. Other fungi provide numerous drugs (such as penicillin and other antibiotics), foods like mushrooms, truffles and morels, and the bubbles in bread (yeast), champagne, and beer. -

Changes in the Human Skin Microbiome Over One Year's Time
American Journal of Microbiology 3 (2): 18-30, 2012 ISSN 1948-982x © 2012 Science Publications doi:10.3844/ajmsp.2012.18.30 Published Online 3 (2) 2012 (http://www.thescipub.com/ajm.toc) Changes in the Human Skin Microbiome Over One Year’s Time 1,2 Richard W. Hyman, 1Farbod Babrzadeh, 1Curtis Palm, 1Chunlin Wang, 1Marilyn Fukushima and 1,2,3 Ronald W. Davis 1Stanford Genome Technology Center, 855 S California Avenue, Palo Alto, CA 94304, USA 2Department of Biochemistry, 3Department of Genetics, Stanford University College of Medicine, Stanford, CA 94305, USA ABSTRACT Human skin comprises a large number of distinguishable ecological niches. To describe fully the human skin microbiome, it will be necessary to identify the bacteria in each niche and to distinguish the commensal bacteria from the temporary residents. To contribute to the description of the human skin microbiome and employing a gene-based technology, we have identified the bacteria in two niches: the front and back of the base of the neck and over the course of one year. There were 50 volunteers and a total of 232 neck skin swabs. Roche 454 Tag pyrosequencing was employed to sequence a short hypervariable sequence region (V6) of the 16S ribosomal RNA gene. To identify the bacteria corresponding to the front and back of the neck for each volunteer, the “Classifier” software in the “Pyrosequencing” section of the Ribosomal Database Project was employed. The bacteria on virtually all 232 neck skin swabs were classified into bacterial Class. The skin microbiome of these two niches was composed principally of a mixture of five Classes of bacteria: Actinobacteria , Alphaproteobacteria , Bacilli , Betaproteobacteria and Gammaproteobacteria . -

Origin of the Cell Nucleus, Mitosis and Sex: Roles of Intracellular Coevolution Thomas Cavalier-Smith*
Cavalier-Smith Biology Direct 2010, 5:7 http://www.biology-direct.com/content/5/1/7 RESEARCH Open Access Origin of the cell nucleus, mitosis and sex: roles of intracellular coevolution Thomas Cavalier-Smith* Abstract Background: The transition from prokaryotes to eukaryotes was the most radical change in cell organisation since life began, with the largest ever burst of gene duplication and novelty. According to the coevolutionary theory of eukaryote origins, the fundamental innovations were the concerted origins of the endomembrane system and cytoskeleton, subsequently recruited to form the cell nucleus and coevolving mitotic apparatus, with numerous genetic eukaryotic novelties inevitable consequences of this compartmentation and novel DNA segregation mechanism. Physical and mutational mechanisms of origin of the nucleus are seldom considered beyond the long- standing assumption that it involved wrapping pre-existing endomembranes around chromatin. Discussions on the origin of sex typically overlook its association with protozoan entry into dormant walled cysts and the likely simultaneous coevolutionary, not sequential, origin of mitosis and meiosis. Results: I elucidate nuclear and mitotic coevolution, explaining the origins of dicer and small centromeric RNAs for positionally controlling centromeric heterochromatin, and how 27 major features of the cell nucleus evolved in four logical stages, making both mechanisms and selective advantages explicit: two initial stages (origin of 30 nm chromatin fibres, enabling DNA compaction; and firmer attachment of endomembranes to heterochromatin) protected DNA and nascent RNA from shearing by novel molecular motors mediating vesicle transport, division, and cytoplasmic motility. Then octagonal nuclear pore complexes (NPCs) arguably evolved from COPII coated vesicle proteins trapped in clumps by Ran GTPase-mediated cisternal fusion that generated the fenestrated nuclear envelope, preventing lethal complete cisternal fusion, and allowing passive protein and RNA exchange. -

Protists – a Textbook Example for a Paraphyletic Taxon
ARTICLE IN PRESS Organisms, Diversity & Evolution 7 (2007) 166–172 www.elsevier.de/ode Protists – A textbook example for a paraphyletic taxon$ Martin Schlegela,Ã, Norbert Hu¨lsmannb aInstitute for Biology II, University of Leipzig, Talstraße 33, 04103 Leipzig, Germany bFree University of Berlin, Institute of Biology/Zoology, Working group Protozoology, Ko¨nigin-Luise-Straße 1-3, 14195 Berlin, Germany Received 7 September 2004; accepted 21 November 2006 Abstract Protists constitute a paraphyletic taxon since the latter is based on the plesiomorphic character of unicellularity and does not contain all descendants of the stem species. Multicellularity evolved several times independently in metazoans, higher fungi, heterokonts, red and green algae. Various hypotheses have been developed on the evolution and nature of the eukaryotic cell, considering the accumulating data on the chimeric nature of the eukaryote genome. Subsequent evolution of the protists was further complicated by primary, secondary, and even tertiary intertaxonic recombinations. However, multi-gene sequence comparisons and structural data point to a managable number of such events. Several putative monophyletic lineages and a gross picture of eukaryote phylogeny are emerging on the basis of those data. The Chromalveolata comprise Chromista and Alveolata (Dinoflagellata, Apicomplexa, Ciliophora, Perkinsozoa, and Haplospora). Major lineages of the former ‘amoebae’ group within the Heterolobosa, Cercozoa, and Amoebozoa. Cercozoa, including filose testate amoebae, chlorarachnids, and plasmodiophoreans seem to be affiliated with foraminiferans. Amoebozoa consistently form the sister group of the Opisthokonta (including fungi, and with choanoflagellates as sister group of metazoans). A clade of ‘plants’ comprises glaucocystophytes, red algae, green algae, and land vascular plants. The controversial debate on the root of the eukaryote tree has been accelerated by the interpretation of gene fusions as apomorphic characters.