Sixth Form Induction
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bibliographie (1) Air Et Cosmos/N°2563/ 29 Septembre 2017 (2) Les
Bibliographie (1) Air et Cosmos/N°2563/ 29 Septembre 2017 (2) Les cahiers de l’Express/N°22/ La conquête de l’espace/Juillet 1993 (3) Les satellites artificiels / Que sais-je ? N°813 / PUF / 1965 (4) Paris-Match / N° 445/ 19 Octobre 1957 (5) Les dossiers du futur/Olivier Orban/Daniel Garric/Décembre 1980 (6) Le Figaro.fr/histoire/archives/02 Novembre 2017 (7) Le livre de l’espace / Denoel / 1978 // (7-1) Le Point/ Hors-Série/ Les chefs d’œuvre de la science-fiction/Novembre-Décembre 2018 (8) La lune et les planètes / Pierre de Latil / 1969 / Collection « Les beaux livres » Hachette (9) Dernières nouvelles du cosmos/ Hubert Reeves/Editions Points/02-2011 (10) Les merveilles du ciel / Guido Ruggieri / 1968 / Collection « Les beaux livres » Hachette (11) Sciences et avenir/N° 850/Décembre 2017 (11-1) Lefigaro.fr/ sciences/ 2018-12-27/01008- 20181227ARTIFIG-decouverte de la plus lointaine planète // (11-2) Sciences et Avenir / N° 864 / Février 2019 (12) Univers / Explorer le monde astronomique/ Phaidon/ Novembre 2017 (13) L’espace pour l’homme / Dominos Flammarion /1993 (14) Le point /N°2348 / 7 Septembre 2017 (15) Sciences et avenir / (16) Sciences et Avenir / N° 858 / Aout 2018 // (16-1) Sciences et Avenir / N° 863 / Janvier 2019 (17) Lefigaro.fr/15-05-2018 (18) Sciences et avenir/N° 846/Aout 2017 (19) Air et Cosmos /N° 835/Septembre 2016 (20) Lefigaro.fr/2018-06-28 (21) Begeek / 25-03-2018 (22) Sciences et Avenir / N° 859 / Septembre 2018 // (22-1) : Le Point/N°2405/4 Octobre 2018// (22- 2) : Air & Cosmos/N° 2620/ 7 Décembre 2018 (23) La -

A Fascinating Literary Puzzle Very Special Souvenirs!
http://enter.lagardere.net www.lagardere.com INNOVATION IS CORE TO OUR BRANDS AND BUSINESSES A fascinating literary puzzle 02 Calmann-Lévy publishes Guillaume Musso’s new novel Very special souvenirs! 03 Lagardère Travel Retail France reappointed to run Eiffel Tower offi cial stores May 2019 #150 PUBLISHING More Stories from Gaul Asterix celebrates his 60th birthday this year, and to mark the occasion, Éditions Albert René are reissuing the very fi rst Asterix book – Astérix le Gaulois – in both luxury and Artbook editions. This anniversary year will culminate in the publication, on October 24, of the 38th book in the Asterix series, with a projected print run of more than 5 mil- © Emanuele Scorcelletti lion copies: La Fille de Vercingétorix. Calmann-Lévy publishes Guillaume Musso’s new novel Late February brought the debut of a new Instagram account, #lArtdAsterix, that offers an inside look at “the art of Asterix”. www.asterix.com A fascinating literary puzzle Guillaume Musso at Hachette Livre island, determined to discover his lated into 34 languages. In July it TRAVEL RETAIL is hitting the headlines twice with secret. That same day, a woman’s will be released as The Reunion the release of La Vie secrète des body is found on a beach and the by Little, Brown in the United States écrivains (Authors’ secret life) and island is cordoned off by the author- and Orion in the UK, and produc- the paperback version of La Jeune ities. Thus begins a dangerous con- tion of a TV adaption for France Fille et la Nuit. frontation between Mathilde and Télévisions is set to start soon. -

Reference Document Including the Annual Financial Report
REFERENCE DOCUMENT INCLUDING THE ANNUAL FINANCIAL REPORT 2012 PROFILE LAGARDÈRE, A WORLD-CLASS PURE-PLAY MEDIA GROUP LED BY ARNAUD LAGARDÈRE, OPERATES IN AROUND 30 COUNTRIES AND IS STRUCTURED AROUND FOUR DISTINCT, COMPLEMENTARY DIVISIONS: • Lagardère Publishing: Book and e-Publishing; • Lagardère Active: Press, Audiovisual (Radio, Television, Audiovisual Production), Digital and Advertising Sales Brokerage; • Lagardère Services: Travel Retail and Distribution; • Lagardère Unlimited: Sport Industry and Entertainment. EXE LOGO L'Identité / Le Logo Les cotes indiquées sont données à titre indicatif et devront être vérifiées par les entrepreneurs. Ceux-ci devront soumettre leurs dessins Echelle: d’éxécution pour approbation avant réalisation. L’étude technique des travaux concernant les éléments porteurs concourant la stabilité ou la solidité du bâtiment et tous autres éléments qui leur sont intégrés ou forment corps avec eux, devra être vérifié par un bureau d’étude qualifié. Agence d'architecture intérieure LAGARDERE - Concept C5 - O’CLOCK Optimisation Les entrepreneurs devront s’engager à executer les travaux selon les règles de l’art et dans le respect des règlementations en vigueur. Ce 15, rue Colbert 78000 Versailles Date : 13 01 2010 dessin est la propriété de : VERSIONS - 15, rue Colbert - 78000 Versailles. Ne peut être reproduit sans autorisation. tél : 01 30 97 03 03 fax : 01 30 97 03 00 e.mail : [email protected] PANTONE 382C PANTONE PANTONE 382C PANTONE Informer, Rassurer, Partager PROCESS BLACK C PROCESS BLACK C Les cotes indiquées sont données à titre indicatif et devront être vérifiées par les entrepreneurs. Ceux-ci devront soumettre leurs dessins d’éxécution pour approbation avant réalisation. L’étude technique des travaux concernant les éléments porteurs concourant la stabilité ou la Echelle: Agence d'architecture intérieure solidité du bâtiment et tous autres éléments qui leur sont intégrés ou forment corps avec eux, devra être vérifié par un bureau d’étude qualifié. -

LONDON Book Fair 2018 Rights List
LONDON Book Fair 2018 Rights List Éditions Calmann-Lévy 21, rue du Montparnasse 75006 Paris FRANCE www.calmann-levy.fr Rights Department Patricia Roussel Rights Director [email protected] +33 (0)1 49 54 36 49 Julia Balcells Foreign & Subsidiary Rights [email protected] +33 (0)1 49 54 36 48 Table of contents Highlights Fiction & Non Fiction • Highlights Fiction Camille Anseaume - Four Walls and a Roof - Quatre murs et un toit •6 Sylvie Baron - Rendezvous in Bélinay - Rendez-vous à Bélinay •7 Boris Bergmann - Apnea - Nage libre •8 Roxane Dambre - An Almost Perfect Karma - Un karma presque parfait •9 Marie-Bernadette Dupuy - Amelia, a Heart in Exile - Amélia, un coeur en exil •10 Johann Guillaud-Bachet - Drowned Alive - Noyé vif •11 Érik L’Homme - Tearing the Shadows - Déchirer les ombres •12 Karine Lambert - Once Upon a Tree - Un arbre, un jour •13 Hélène Legrais - The Angels of Beau-Rivage - Les anges de Beau-Rivage •14 Alfred Lenglet - Hearts of Glass - Coeurs de glace •15 Antonin Malroux- The Straw-made Bread - Le pain de paille •16 Éric Le Nabour - Back to Glenmoran - Retour à Glenmoran •17 Florence Roche - Philomena and her kin - Philomène et les siens •18 Julien Sandrel - The Room of Wonders - La Chambre des merveilles •19 Jean Siccardi - Stepping Stone Inn - L’auberge du Gué •20 Laurence Peyrin - The Virgins’ Wing - L’aile des vierges •21 Pascal Voisine - My Kid - Mon Gamin •22 • Highlights Suspense Fiction Jérôme Loubry - The Hounds of Detroit - Les chiens de Detroit •24 Philippe Lyon -The Black Piece - L’oeuvre noire -

The Color of May 1968. Paris Match and the Events of May and June 1968 Audrey Leblanc
The Color of May 1968. Paris Match and the Events of May and June 1968 Audrey Leblanc To cite this version: Audrey Leblanc. The Color of May 1968. Paris Match and the Events of May and June 1968. Etudes photographiques, Société française de photographie, 2010. hal-01395719 HAL Id: hal-01395719 https://hal.archives-ouvertes.fr/hal-01395719 Submitted on 11 Nov 2016 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. The Color of May 1968 of black and white photography – unusual for the magazine, which nor- Paris Match and the Events mally published in color – tells the story of May and June 1968 of the obstacles its editors faced in designing and producing the magazine. An analysis of the choices the magazine yndicat du Livre (the Printers’ made reveals the economic and ideo- SUnion) joined the call that went out logical issues that came into play in for a general strike and mass demonstra- covering the events of May 1968. tion to be held on May 13, 1968. Most of the workers in the printing plants The Magazine’s Editorial and paper industries were members of Conventions Overturned this union, leaving the editors of the In early May 1968, the entire French French news magazines L’Express, Le Nou- press reacted sharply to the dramatic vel Observateur, and Paris Match with confrontations between students and immediate disruptions of their printing police that had taken place on May 6 at and distribution. -

The Association for Diplomatic Studies and Training Foreign Affairs Oral History Project
The Association for Diplomatic Studies and Training Foreign Affairs Oral History Project PHILIP C. BROWN Interviewed by: Charles Stuart Kennedy Initial interview date: January 18, 2012 Copyright ADST 2016 TABLE OF CONTENTS Background Born in Massachusetts; raised primarily in Pennsylvania College of Wooster, Ohio; Fletcher School of Law and Diplomacy Operation Crossroads Africa Marriage Washington, DC; Voice of America; Africa news room 1965 Entered the Foreign Service, USIA 1965 State Department: Foreign Service Institute (FSI): 1965 French language training Dakar, Senegal: USIA: Junior Officer Trainee 1966-1967 President Leopold Senghor French presence Lebanese Festival of Negro Arts John McKesson Ambassador William R. Rivkin Environment Cultural Center operations Recreation Islam Douala, Cameroon: Branch Public Affairs Officer 1967-1968 Environment Cultural Center operations Religions French “cooperants” French influence Institute of International Education Biafra War 1 Voice of America Birth of daughter Yaoundé, Cameroon: Cultural Affairs Officer 1968-1970 Environment President Ahmadou Ahidjo Tribal influence French presence Relations Political climate Ambassador Robert Payton Embassy staff Living arrangements Jim Bishop Recreation Visitors Program Ambassador Lewis Hoffacker Secretary and Mrs. Rogers visit Algiers, Algeria: Cultural Affairs Officer 1970-1972 American Interests Section, Embassy of Switzerland 1967 Six Day War Economic relations Political relations US Export-Import Bank loans El Paso Natural Gas William Eagleton Scholarship/Visitors’ -

English Department Things to Read
Sixth Form Wider Reading and Discovery Lists for A- Level Subjects SIXTH FORM Mindset by Carol Dweck. Brain Rules by John Medina Outliers – The story of success by Malcolm Gladwell Bounce: The Myth of talent and the power of practice by Matt Syed The Tipping Point:How little things can make a big difference by Malcolm Gladwell. Art and Design Books The Andy Warhol Diaries Edited by Pat Hackett pub by Warner Books The American Leonardo: A Tale of 20th Century Obsession, Art and Money by John Brewer I Was Vermeer: The Forger Who Swindled the Nazis by Frank Wynne Art and Fear: Observations on the Perils (and Rewards) of Artmaking by David Bayles How to Survive and Prosper as an Artist-Selling yourself without Selling your Soul by Caroll Michels Artist's Guide to Selling work by Annabelle Ruston This is Modern Art by Matthew Collings Inside the White Cube: The Ideology of the Gallery Space by Brian O'Doherty Ways of Seeing by John Berger Understanding and Investigating Art by Rod Taylor pub by Hodder and Stoughton Dictionary of Subjects and Symbols in Art by James Hall Art Now (vol 3 ) by Hans Werner Holzwarth pub by Taschen Drawing on the Right side of the Brain by Betty Edwards pub by Souveneir Press (think has a new publisher now) The Thames and Hudson Dictionary of Art and Artists by Herbert Read and Nikos Stangos AS/A level Art and Design Essential Word dictionary by Mark White Book - The Shock of the New, by Robert Hughes Book/DVD - The Power of Art Arteffects by Jean Drysdale Green The Encyclopedia of Acrylic Techniques by Hazel Harrison Sources of Inspiration for Ceramic and the Applied Arts by Carolyn Genders Magazines/Journals Modern Painters -brilliant monthly magazine devoted to painting, only interviews with painters and exhibition reviews. -

2019 Universal Registration Document CHAPTER 1 - Overview of the Group
UNIVERSAL REGISTRATION DOCUMENT including the Annual Financial Report FISCAL YEAR 2019 ProfilE Created in 1992, Lagardère is an international group with operations in more than 40 countries worldwide. It employs over 30,000 people and generated revenue of €7,211 million in 2019. Under the impetus of the Group’s General and Managing Partner, Arnaud Lagardère, the Group launched a strategic refocusing around two priority divisions: Lagardère Publishing is the world’s third-largest Lagardère Travel Retail, is the world’s fourth book publisher for the general public and largest travel retail merchant, with operations educational markets, and the leader in France. in three segments of this very dynamic field: Alongside some 6,900 employees, it creates Travel Essentials, Duty Free & Fashion, and 17,000 original publications each year as well Foodservice. Lagardère Travel Retail has as contributing to their broader circulation 25,000 employees across an international by innovating with digital and mobile reading network of more than 4,800 points of sale formats. Lagardère Publishing’s activities also in around one thousand airports, mainline extend to adjacent businesses such as Mobile and urban train stations. Games and Board Games. The Group’s business scope also includes Lagardère News (Paris Match, Le Journal du Dimanche, Europe 1, RFM, Virgin Radio and the Elle brand licence) together with Lagardère Live Entertainment. The Lagardère Studios unit is in the process of being sold. Through this strategic refocusing, the Lagardère group is investing in its two strategic divisions with the aim of creating global leaders over the long term. Lagardère shares are listed on Euronext Paris. -

Doc De Ref 2005M
Reference Document 2005 5_1 Lagardère SCA - Business activities and strategy .................................32 5_2 The Group's principal activities and main markets – Operations in the course of 2005.................................................................35 5_2_1 Media operations ..........................................................................................................35 5_2_1_1 Book Publishing ..............................................................................................................35 5_2_1_2 Print Media ....................................................................................................................41 5_2_1_3 Distribution Services ........................................................................................................47 5_2_1_4 Lagardere Active .............................................................................................................52 5_2_2 High Technologies .........................................................................................................61 5_2_3 Other business activities ...............................................................................................75 5_3 Labour and environmental information – Business ethics ......................77 5_3_1 Labour information .......................................................................................................78 5_3_2 Corporate citizenship ....................................................................................................99 5_3_3 -
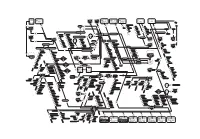
Mediaindustrialcomplex2002
deutsche bank saint philips financière groupe 50 franklin gobain putnam prudential enderson pinault arnault ressources century inv. investors fendi 51 sport-informations dienst 5 6,3 4 4,78 rvp 99 12 sofiee polycom aurora ipsos 62 3,4 daimler ipsos compagny news delor s.a. le point chrysler 14,9 europ@web 70 44 libertysurf 33,9 nolis finintel château latour inedit ysl beauté conforama 100 44 holding canal + sefimeg france printemps a christian dior sa 2,74 i 1,11 d artprice.com usa networks agence christie s vivendi e ' fnac 44 mdo monte carlo 40 france canal satellite presse famille univers. m paris première kiosque bronfman lagardère studio participations 40 6,1 sa eso tkp (poland) artemis pinault printemps lvmh king canal jimmy la redoute lagardère c : canal + (flandres) fisher sca famille seasons tv sport tele + (italia) verrechia havas havas advertising 3,7 pathe 50 eurochannel comédie ! vox (germany) euro rscg planete investir game one canal + (pays bas) fleuve noir hachette sa virgin arnold worldwide partn. bouyghes ciné classics tribune desfossés demain ! (spain) prada sogecable 42,5 10/18 mediaplanning 15,5 agefi ciné cinéma i-télévision canal + (africa) pocket mediametrie tf 1 p 39,3 u bl ugc 6 is hachette livres hachette filipacchi julliard h . sotheby's t él robert laffont chaîne festival év i 27 sion multi thematiques calmann-levy elle étude Tojan comareg à euronews péage rmc disney hachette ed. journal du dimanche philips (art auction) l'expansion tv 5 7 9 fayard parents l l étudiant v ' sci fr. télévis. French c ap i t la sentinelle télé 7 jours al state of havas images l'express chaînes région State grasset ici paris france vodafone benefits cosmetics kuweit courrier international cinéma 4 million telecom 4,3 18 harlequin rapho images 50 vizzavi 50 la cinquième hard candy havas business ed. -

Yahoo! on Trial (A)
INSEAD Business e-Ethics: Yahoo! on Trial (A) 09/2001-4956 This case was prepared by Dr. Mark Hunter, Senior Research Fellow at INSEAD, under the supervision of Marc Le Menestrel, Assistant Professor of Economics and Business at University Pompeu Fabra and Visiting Professor at INSEAD, and Henri-Claude de Bettignies, Professor of Asian Business at INSEAD and Visiting Professor at Stanford University. It is intended to be used as a basis for class discussion rather then to illustrate either effective or ineffective handling of an administrative situation. Copyright © 2001 INSEAD, Fontainebleau, France. N.B. PLEASE NOTE THAT DETAILS OF ORDERING INSEAD CASES ARE FOUND ON THE BACK COVER. COPIES MAY NOT BE MADE WITHOUT PERMISSION. INSEAD 1 4956 Introduction: Calling Jerry Yang On a fine day in June 2000, Jerry Yang, the co-founder of Yahoo!, arrived in the Paris offices of his company’s French subsidiary. The previous evening, fresh from a conference in London sponsored by Fortune magazine where he was among the star speakers, he had joined in the inaugural bash for Yahoo! France’s new building in an elegant quarter of Paris. Upholstered in Yahoo!’s purple and orange team colors with a soda machine offering free drinks, and five-point yellow stars bearing the names of new employees on the walls, the place evokes a magically perfect American high school, except that the youthful employees work very fast, long and hard. As Yang settled in, a reporter for one of France’s major newspapers, Libération, called to request an interview on a painfully sensitive subject. -

Claiming Les Halles: Architecture and the Right to the City
809 Claiming Les Halles: Architecture and the Right to the City ROBERT WEDDLE Drury University In 1985, Jacques Chirac, then mayor of Paris, as- sisted in the inauguration of what seemed at the time the fi nal step in the long and complicated effort to renovate the Les Halles quarter in cen- tral Paris, which for centuries housed the city’s fresh food markets. The day marked, for Chirac, “the fi nal act in the most signifi cant urban opera- tion undertaken in Paris in decades.”1 Others in- volved in the project echoed Chirac’s sense of fi - nality. Three years later, the semi-public company founded to oversee the quarter’s transformation was dissolved; the group’s president, sociologist and politician Christian de la Malène, declared the work at Les Halles completed.2 But de la Malène’s statements were tempered by a recognition of the contingent nature of planning. “Parisians,” he pre- Les Halles, Rue Lescot, 2007 dicted, … have not only appointed themselves censors of But the efforts of Delanöe, as well as the designs the project; they will in a thousand ways be the of Mangin and others, have been subjected to dynamic creators of tomorrow. Over the months and years the trees will grow and the facades will withering criticism in the press and, especially, patina, and the inhabitants of Paris will give the from neighborhood groups representing inhabit- quarter its role and its face. In the end, Les Halles ants, shopkeepers, and business people who have 3 will only be what Parisians make of it.