The Effects of Thinning on Forest-Floor Small Mammals in the Coastrange of Oregon
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

OAEC Wildlife List
Vertebrate Species of the Occidental Arts and Ecology Center * Indicates that species has not been positively sighted to date, but is expected to occur. This is a list of animals actually sighted by Brock Dolman from 7/31/94 to date. KINGDOM: ANIMALIA PHYLUM: VERTEBRATA CLASS: AMPHIBIA ORDER: CAUDATA (Salamanders) FAMILY: DICAMPTODONTIDAE (Mole Salamanders and Relatives) California Giant Salamander (Dicamptodon ensatus) FAMILY: SALAMANDRIDAE (Newts) California Newt (Taricha torosa) FAMILY: PLETHODONITDAE (Lungless Salamanders) Ensatina (Ensatina eschscholtzi) California Slender Salamander (Batrachoseps attenuatus) Arboreal Salamander (Aneides lugubris) * Black Salamander (Aneides flavipunctatus) ORDER: SALIENTIA (Frogs and Toads) FAMILY: HYLIDAE (Treefrogs and Relatives) Pacific Treefrog (Hyla)(Pseudacris regilla) FAMILY: RANIDAE (True Frogs) Bullfrog (Rana catesbeiana) CLASS: REPTILIA ORDER: TESTUDINES (Turtles) FAMILY: EMYDIDAE (Pond and Marsh Turtles) Western Pond Turtle (Clemmys marmorata) Pond Slider (Trachemys scripta) Introduced ORDER: SQUAMATA (Lizards and Snakes) SUBORDER: SAURIA (Lizards) FAMILY: IGUANIDAE (Iguanids) Northwestern Fence Lizard (Sceloporus occidentalis occidentalis) FAMILY: SCINCIDAE (Skinks) Western Skink (Eumeces skiltonianus skiltonianus) FAMILY: ANGUIDAE (Alligator Lizards and Relatives) Northern Alligator Lizard (Gerrhonotus coeruleus) Sub-species ?? *Southern Alligator Lizard (Gerrhonotus multicarinatus multicarinatus) SUBORDER: SERPENTES (Snakes) FAMILY: BOIDAE (Boas) Rubber Boa (Charina bottae bottae) FAMILY: -

Natural History of Oregon Coast Mammals Chris Maser Bruce R
Forest Servile United States Depa~ment of the interior Bureau of Land Management General Technical Report PNW-133 September 1981 ser is a ~ildiife biologist, U.S. ~epa~rn e Interior, Bureau of La gement (stationed at Sciences Laboratory, Corvallis, Oregon. Science Center, ~ewpo Sciences Laborato~, Corvallis, Oregon. T. se is a soil scientist, U.S. wa t of culture, Forest Service, Pacific rthwest Forest and ange ~xperim Station, lnst~tute of orthern Forestry, Fairbanks, Alaska. Natural History of Oregon Coast Mammals Chris Maser Bruce R. Mate Jerry F. Franklin C. T. Dyrness Pacific Northwest Forest and Range Experiment Station U.S. Department of Agriculture Forest Service General Technical Report PNW-133 September 1981 Published in cooperation with the Bureau of Land Management U.S. Department of the Interior Abstract Maser, Chris, Bruce R. Mate, Jerry F. Franklin, and C. T. Dyrness. 1981. Natural history of Oregon coast mammals. USDA For. Serv. Gen. Tech. Rep. PNW-133, 496 p. Pac. Northwest For. and Range Exp. Stn., Portland, Oreg. The book presents detailed information on the biology, habitats, and life histories of the 96 species of mammals of the Oregon coast. Soils, geology, and vegetation are described and related to wildlife habitats for the 65 terrestrial and 31 marine species. The book is not simply an identification guide to the Oregon coast mammals but is a dynamic portrayal of their habits and habitats. Life histories are based on fieldwork and available literature. An extensive bibliography is included. Personal anecdotes of the authors provide entertaining reading. The book should be of use to students, educators, land-use planners, resource managers, wildlife biologists, and naturalists. -

Talpid Mole Phylogeny Unites Shrew Moles and Illuminates Overlooked Cryptic Species Diversity Kai He,‡,†,1,2 Akio Shinohara,†,3 Kristofer M
Talpid Mole Phylogeny Unites Shrew Moles and Illuminates Overlooked Cryptic Species Diversity Kai He,‡,†,1,2 Akio Shinohara,†,3 Kristofer M. Helgen,4 Mark S. Springer,5 Xue-Long Jiang,*,1 and Kevin L. Campbell*,2 1State Key Laboratory of Genetic Resources and Evolution, Kunming Institute of Zoology, Chinese Academy of Sciences, Kunming, China 2Department of Biological Sciences, University of Manitoba, Winnipeg, MN , Canada 3Department of Bio-resources, Division of Biotechnology, Frontier Science Research Center, University of Miyazaki, Miyazaki, Japan 4National Museum of Natural History Smithsonian Institution, Washington, DC 5Department of Biology, University of California, Riverside, CA ‡Present address: The Kyoto University Museum, Kyoto University, Kyoto, Japan †These authors contributed equally to this work. *Corresponding authors: E-mails: [email protected]; [email protected] Associate editor: Emma Teeling Abstract The mammalian family Talpidae (moles, shrew moles, desmans) is characterized by diverse ecomorphologies associated with terrestrial, semi-aquatic, semi-fossorial, fossorial, and aquatic-fossorial lifestyles. Prominent specializations involved with these different lifestyles, and the transitions between them, pose outstanding questions regarding the evolutionary history within the family, not only for living but also for fossil taxa. Here, we investigate the phylogenetic relationships, divergence times, and biogeographic history of the family using 19 nuclear and 2 mitochondrial genes (16 kb) from 60% of described species representing all 17 genera. Our phylogenetic analyses help settle classical questions in the evolution of moles, identify an ancient (mid-Miocene) split within the monotypic genus Scaptonyx, and indicate that talpid species richness may be nearly 30% higher than previously recognized. Our results also uniformly support the monophyly of long-tailed moles with the two shrew mole tribes and confirm that the Gansu mole is the sole living Asian member of an otherwise North American radiation. -

Influence of Alternative Silviculture on Small Mammals
Influence of Alternative Silviculture on Small Mammals Background 3. if path selection by Townsend’s chipmunk is influenced by downed wood. Small mammals are an important component of forest ecosystems in the Pacific Northwest and are the primary prey This study was located in the McDonald-Dunn Research for many species of predators, such as the northern spotted owl Forest in the Oregon Coast Range (Figure 1). The Douglas-fir- and marten. In addition, small mammals play key roles in the dominated stands in this study were 30–90 years old and were dispersal of fungal spores and influence population dynamics of part of a long-term research project with uncut control stands herbaceous and woody plants. and replicates of three alternative silvicultural treatments imple- A variety of silvicultural methods (e.g., thinning, green-tree mented from 1989 to 1991. From least to most intense, the three retention) can be used as alternatives to traditional clearcut treatments were small group-selection (where 33% of the stand systems to promote structural diversity within forest stands and volume is removed in patches), two-story (75% of the volume to provide commodity production. However, our understanding is uniformly removed), and clearcut. For their first objective, of the responses of wildlife in general, and small mammals in Waldien and Hayes sampled the small mammal population in particular, to these methods is poorly developed. two replicates of In recent years, there has been increased interest in retaining each stand McDonald-Dunn Forest dead wood on the forest floor to promote biodiversity and other Coast Range ecological values. -

SHREWS Robert H
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln The aH ndbook: Prevention and Control of Wildlife Wildlife Damage Management, Internet Center for Damage 1-1-1994 SHREWS Robert H. Schmidt Utah State University, [email protected] Follow this and additional works at: http://digitalcommons.unl.edu/icwdmhandbook Part of the Environmental Sciences Commons Schmidt, Robert H., "SHREWS" (1994). The Handbook: Prevention and Control of Wildlife Damage. 55. http://digitalcommons.unl.edu/icwdmhandbook/55 This Article is brought to you for free and open access by the Wildlife Damage Management, Internet Center for at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in The aH ndbook: Prevention and Control of Wildlife Damage by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Robert H. Schmidt Assistant Professor Department of Fisheries and Wildlife SHREWS Utah State University Logan, Utah 84322-5210 Fig. 1. A masked shrew, Sorex cinereus Identification Damage Prevention and Fumigants The shrew is a small, mouse-sized Control Methods None are registered. mammal with an elongated snout, a Trapping dense fur of uniform color, small eyes, Exclusion and five clawed toes on each foot (Fig. Mouse trap (snap trap). Rodent-proof structures also exclude 1). Its skull, compared to that of shrews. Small box trap. rodents, is long, narrow, and lacks the zygomatic arch on the lateral side Cultural Methods Pit trap. characteristic of rodents. The teeth are Mowing may decrease preferred Shooting small, sharp, and commonly dark- tipped. Pigmentation on the tips of the habitat and food. Not practical. teeth is caused by deposition of iron in Repellents Other Methods the outer enamel. -

Mammal Inventory at Oregon Islands National Wildlife Refuge, Crook
Mammal Inventory Report Crook Point, Oregon, 2009. Siskiyou Research Group Introduction and Study Area The Crook Point Unit of Oregon Islands National Wildlife Refuge is a 134-acre undeveloped coastal headland located approximately 12 miles south of Gold Beach, Oregon. Notwithstanding post-European settlement activities of livestock grazing and timber harvesting, Crook Point provides exceptional examples of coastal headland habitats, which have largely disappeared from Oregon as a result of development and conversion (Kagan 2002). Currently, Crook Point provides habitat for rare plant species such as Poa unilateralis, Lasthenia ornduffii, and Artemisia pycnocephala (Kagan 2002, Chan 2001, Bilderback 2008). Moreover, the associated offshore islands and rocks provide important nesting habitat for seabirds, haul-out and breeding areas for marine mammals, and suitable cliff habitat for nesting peregrine falcons (Ledig 2009). Saddle Rock, the largest island associated with Crook Point, provides nesting habitat for a large population of Leach’s storm petrels (PRBO 2004). There is evidence the Saddle Rock population is being decimated by mammalian predators (reference). In 2000 U.S. Fish and Wildlife Service (USFWS) acquired this coastal headland making it the second mainland unit in the Oregon Islands National Wildlife Refuge system. Presently USFWS is focusing on site restoration, resource protection, and biological inventory, monitoring, and research. This mammal survey is part of an effort to document the biological diversity of Crook Point. Prior to this survey no systematic mammal inventory work has been conducted at Crook Point. Objectives The primary objective of this survey was to compile a mammalian species list for Crook Point using multiple detection methods of trapping, motion-triggered trail cameras, direct observation, and animal sign (prints, scat, excavations). -

Washington Species List
TheThe NatureMappingNatureMapping ProgramProgram Washington Cooperative Fish and Wildlife Research Unit School of Fisheries and Aquatic Sciences University of Washington Species of Washington • Amphibians • Reptiles • Fish • Birds • Mammals For more information on NatureMapping http://depts.washington.edu/natmap last update: 01/03/06 The NatureMapping Program Amphibians (27 species) Frogs/Toads (13 species) RA99 Unidentified Rana Rana sp. RACAT Bullfrog Rana catesbeiana RANCA Cascades frog Rana cascadae SPIN Great basin spadefoot Scaphiopus intermontana RACL Green frog Rana clamitans RAPI Northern leopard frog Rana pipiens PSRE Pacific treefrog (Chorus frog) Hyla regilla RAAU Red-legged frog Rana aurora RALU Spotted frog (Eastside) Rana luteiventris RAPR Spotted frog (Westside) Rana pretiosa ASTR Tailed frog Ascaphus truei BUBO Western toad Bufo boreas RASY Wood frog Rana sylvatica BUWO Woodhouse's toad Bufo woodhousei Salamanders/Newts (14 species) AM99 Unidentified Ambystoma Ambystoma sp. RHCAS Cascade torrent salamander Rhyacotriton cascadae RHKE Columbia torrent salamander Rhyacotriton kezeri DICO Cope's giant salamander Dicamptodon copei PLDU Dunn's salamander Plethodon dunni ENES Ensatina Ensatina eschscholtzi PLLA Larch mountain salamander Plethodon larselli AMMA Long-toed salamander Ambystoma macrodactylum AMGR Northwestern salamander Ambystoma gracile RHOL Olympic torrent salamander Rhyacotriton olympicus DITE Pacific giant salamander Dicamptodon tenebrosus TAGR Roughskin newt Taricha granulosa AMTI Tiger salamander Ambystoma -

The Butte Research Natural Area
THE BUTTE RESEARCH NATURAL AREA Supplement 261 Sarah E. Greene and Larry R. Scofield2 The Research Natural Area described in this Of the 105 Federal Research Natural Areas supplement is administered by the Bureau of established in Oregon and Washington, 45 are Land Management, U.S. Department of the Inte- described in "Federal Research Natural Areas in rior. Bureau of Land Management Research Nat- Oregon and Washington: A Guidebook for Scien- ural Areas are administered by District Offices tists and Educators" (see footnote 1). Supple- that are organizational subdivisions of their State ments to the guidebook describe additions to the Offices. Scientists wishing to use these Research system. Natural Areas (RNA) in Oregon and Washington The guiding principle in management of should contact the District Manager of the district Research Natural Areas is to prevent unnatural in which the Research Natural Area is located. encroachments or activities that directly or indi/ The Butte Research Natural Area is part of a rectly modify ecological processes. Logging and Federal system of such tracts established for uncontrolled grazing are not allowed, for exam- research and educational purposes. Each RNA ple, nor is public use that might impair scientific constitutes a site where natural features are pre- or educational values. Management practices served for scientific purposes and natural proc- necessary for maintenance of ecosystems may be esses are allowed to dominate. Their main allowed. purposes are to provide: Federal Research Natural Areas provide a Baseline areas against which effects of unique system of publicly owned and protected human activities can be measured; examples of undisturbed ecosystems where scien- Sites for study of natural processes in undis- tists can conduct research with minimal inter- turbed ecosystems; and ference and reasonable assurance that 3. -
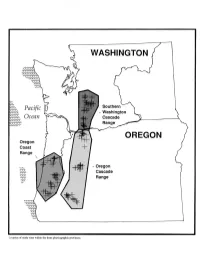
Regional Patterns of Small Mammal Abundance and Community Composition in Oregon and Washington
Regional Patterns of Small Mammal Abundance and Community Composition in Oregon and Washington Authors Ordination analysis of small mammal communities, both KEITH B. AUBRY is a research wildlife biologist and 1 among and within provinces, revealed few ecologically MARK J. CRITES is a wildlife biologist, USDA Forest interpretable patterns or differences among forest age- Service, Pacific Northwest Research Station, Olympia, classes. Most variation was attributable to differences in Washington 98502; STEPHEN D. WEST is an associate species composition resulting from zoogeographic barriers. professor, College of Forest Resources, University of The shrew-mole and red tree vole were closely associated Washington, Seattle, Washington 98195. with old-growth forests in all provinces where they occurred. These species should be given high priority in the develop- Abstract ment of management strategies for small mammals in Pacific Pitfall traps were used by a team of investigators to study Northwest forests. forest-floor small mammal communities in 144 different- aged Douglas-fir forest stands in three physiographic prov- Introduction inces in the Pacific Northwest: the Oregon Coast Range, The fate of old-growth Douglas-fir forests in the Pacific Oregon Cascade Range, and Southern Washington Cascade Northwest has become one of the most controversial re- Range. All three small mammal assemblages were numer- source management issues of this century (see Norse 1990, ically dominated by a few species. The Trowbridge’s shrew Norse and others 1986, Wilcove 1988). Research on the com- was the most abundant species in all three provinces and position, structure, and function of these forests, however, accounted for 47 percent of 8661 small mammals captured. -
Checklist of North American Mammals North of Mexico
OCCASIONAL PAPERS THE MUSEUM TEXAS TECH UNIVERSITY NUMBER 12 2 FEBRUARY 1973 CHECKLIST OF NORTH AMERICAN MAMMALS NORTH OF MEXICO J. KNOX JONES, JR., DILFORD C. CARTER, AND HUGH H. GENOWAYS Since the publication of "Vernacular names of North American mammals north of Mexico" by Hall et al. (1957) and its subsequent revision (Hall, 1965), systematic and other studies have contributed materially to a better understanding of the nomenclature of North American mammals. For this reason, and because of the usefulness, principally to students, of checklists that record both scientific and vernacular names, an up-dated listing of New World mammalian species north of Mexico is timely. As in the previous lists, a specific vernacular name applies to all populations of that species. The present checklist includes all species of North American mam mals recognized as of the close of 1972 and is based on the relevant published literature. Insofar as practicable, we have avoided personal taxonomic judgements. By way of example, some recent studies re flected in the list include those of Mitchell (1968), who reduced the Odobenidae to subfamilial status under Otariidae, van Zyll de Jong (1972), who separated New World otters from those of the Old World under the generic name Lontra, and Lee et al. (1972), who elevated to specific status Peromyscus attwateri and P comanche. On-going sys tematic studies will result in fiiture additions and deletions to the list; this is particularly true for the Cetacea, where our provisional arrange ment follows closely that proposed by Rice and Scheffer (1968). Various species of non-native mammals have been introduced into North America over the years—both accidentally, as in the case of Old World murid rodents, or for some purpose, as in the case of un gulates imported as big game. -

Mammal Species Native to the USA and Canada for Which the MIL Has No Image (170) 31 July 2021
Mammal species native to the USA and Canada for which the MIL has no image (170) 31 July 2021 ARTIODACTYLA (includes CETACEA) (25) BALAENIDAE - bowheads and right whales 1. Eubalaena japonica - Northern Pacific Right Whale 2. Eubalaena glacialis – North Atlantic Right Whale BALAENOPTERIDAE - rorqual whales Balaenoptera ricei - Rice’s Whale DELPHINIDAE - ocean dolphins 1. Feresa attenuata - Pygmy Killer Whale 2. Globicephala melas - Long-finned Pilot Whale 3. Lagenodelphis hosei - Fraser’s Dolphin 4. Leucopleurus acutus - Atlantic White-sided Dolphin 5. Stenella attenuata - Pantropical Spotted Dolphin 6. Stenella clymene - Clymene Dolphin 7. Stenella longirostris - Spinner Dolphin KOGIIDAE - pygmy sperm whales 1. Kogia breviceps - Pygmy Sperm Whale 2. Kogia sima - Dwarf Sperm Whale ZIPHIIDAE - beaked whales 1. Berardius bairdii - Baird’s Beaked Whale 2. Berardius minimus - Least Beaked Whale 3. Hyperoodon ampullatus - Northen Bottlenose Whale 4. Indopacetus pacificus - Tropical Beaked Whale 5. Mesoplodon bidens - Sowerby’s Beaked Whale 6. Mesoplodon carlhubbsi – Hubbs’s Beaked Whale 7. Mesoplodon densirostris - Blainville’s Beaked Whale 8. Mesoplodon europaeus - Gervais’s Beaked Whale 9. Mesoplodon ginkgodens - Ginkgo-toothed Beaked Whale 10. Mesoplodon mirus - True’s Beaked Whale 11. Mesoplodon perrini - Perrin’s Beaked Whale 12. Mesoplodon stejnegeri - Stejneger’s Beaked Whale 13. Ziphius cavirostris - Cuvier’s Beaked Whale CARNIVORA (8) CANIDAE - dogs Canis lycaon - Eastern Wolf MEPHITIDAE - skunks 1. Conepatus leuconotus - American Hog-nosed Skunk 2. Spilogale gracilis - Western Spotted Skunk MUSTELIDAE - weasels and relatives Martes americana - American Marten OTARIIDAE - eared seals Arctocephalus townsendi - Guadalupe Fur Seal PHOCIDAE - earless seals 1. Cystophora cristata - Hooded Seal 2. Histriophoca fasciata - Ribbon Seal 3. Phoca largha - Spotted Seal CHIROPTERA (20) MOLOSSIDAE - free-tailed bats 1. -

A List of Amphibians, Reptiles, Birds and Mammals California
A list of amphibians, reptiles, birds and mammals California Edited By William F. Laudenslayer Jr. and William E. Grenfell Ir. This list is a product of the the basis of the reviewers' cor- must be emphasized that the list is California Wildlife Relationshim rections and comments. not static; it will change over time Program and the ~alifornia Scientific and English names as new information is acquired. Interagency Wildlife Task Group. also were- selected from various New species may be added, or listed Objectives of the effort were to: publications. Scientific names species may be deleted based on Establish a list of all selected were those which appear to new information. Names also may amphibians, reptiles, birds and be most correct at this time. be changed based on new mammals which occur in English names selected included information which suggests that a California for the purpose of those which were most appropriate species more accurately should be developing species-habitat rela- for California. For birds, this placed in a different genus, two tionships models (Marcot 1979, exercise required examination of species probably are two Airola 1980, Osaki 1980, Salwasser the American Ornithologists' subspecies of the same species, or et al. 1980, Verner and Boss 1980, Union Checklist (AOU 1957) and two subspecies probably are one Grenfell and Salwasser 1981, supplements (AOU 1973, 1976). species. As a result of such Grenfell et al. 1982, Laudenslayer The 1957 checklist is in the process fluctuations in vertebrate 1982, Loe and Keeney 1983) for use of being rewritten, so additional taxonomy, this list will have to be by management agencies, and material (Jones et al.