Clinical Thermometers
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

MASTER PLAN DOCUMENT.Indd
Master Plan for the RICHARDSON OLMSTED COMPLEX Buffalo, NY September 2009 9.29.09 Prepared For: THE RICHARDSON CENTER CORPORATION By: CHAN KRIEGER SIENIEWICZ CKS ARCHITECTURE AND URBAN DESIGN in collaboration with: Richardson Center Corporation (RCC) Richardson Architecture Center, Inc Reed Hilderbrand *Stanford Lipsey, Chairman Peter J. Atkinson - Capital Projects Manager, Landscape Architecture Publisher, The Buffalo News Harvard University Art Museums Watertown, MA *Howard Zemsky, Vice Chairman Anthony Bannon - Director, Urban Design Project President, Taurus Capital Partners, LLC. George Eastman House Public Process URBAN DESIGN PROJECT Buffalo, NY *Christopher Greene, Secretary Barbara A. Campagna, FAIA, LEED AP - Graham Gund Architect of the Partner, Damon & Morey, LLP National Trust for Historic Preservation City Visions/ City Properties Real Estate Development *Paul Hojnacki, Treasurer Brian Carter, Ex Offi cio - Dean and Professor, Louisville, KY President, Curtis Screw Company University at Buffalo School of Architecture and Planning Clarion Associates Carol Ash, Commissioner Louis Grachos - Director, Economic Modeling NYS Offi ce of Parks, Recreation, and Historic Preservation Albright-Knox Art Gallery Chicago, Il *Clinton Brown, President Robert Kresse – Attorney, Parsons Brinckerhoff Clinton Brown Co. Architecture, PC Hiscock & Barclay, LLP Permitting Buffalo, NY Paul Ciminelli, President & CEO Lynn J. Osmond - President and CEO, Ciminelli Development Company Chicago Architecture Foundation Bero Architecture Historic Preservation -

Out-Patient Clinics for Nervous and Mental Diseases in the United States ·
Out-Patient Clinics for Nervous and Mental Diseases in the United States · Prepared by 0. J . D' ALTON, M.D . .\ _,. Special Assistant, Reconstruction War Work, The National Committee for Mental Hl'liene THE NATIONAL COMMITTEE FO.R MENTAL HYGmNE, Inc. 50 U n i o n S q u a r e New York• City 1910 OUT-PATIENT CLINICS FOR NERVOUS AND MENTAL DISEASES IN THE UNITED STATES Prepared by ----- C. J . D'ALTOX, l\f.D. Special. Assistwnt, Reconstnwtion Wll!r T-1"1o1·k, The Xational Oommittee for · M ental Hyg·iene CLINICS IN DISTRICT NUMBER 1 * Maine Massachusets-Continuecl Augusta.-Out-Patient Clinic of the Bo'ston.-Out-Patient Clinic of the Bos Augusta State Hospital. ton State Hospital. Held at the hospital. Held at the P sychopathic Depart- Director: Forest C. Tyson, :M.D., ment, 74 Fenwood Road. Superintendent of the Augusta State Director: Percy L. Dodge, M.D. Hospital. Mental and neurological patients. Mental and neurological patients. Hours: Daily, except Sunday and Hours: By appointment. holidays, 2 to 4 p. m. Bangor.-Free P sychopathic Clinic of the Bangor State Hospital. Out-Patient Clinic of the Boston Held at City Hall, Room 7. City Hospital. Director: Lester F. Norris, M.D., Held at 818 Harrison AYenue. Superintendent of the Bangor State Director: Philip C. Knapp, M.D. Hospital. Mental and neurological patients. Mental and neurological patients. Hours: Daily, except Sund.ay, Hours: First and t hird Saturdays 8:30 to 10:30 a.m. of each month , 1:30 to 3:30 p. m. -
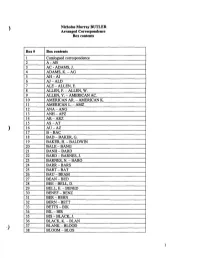
Nicholas Murray BUTLER Arranged Correspondence Box Contents Box
Nicholas Murray BUTLER Arranged Correspondence Box contents Box# Box contents 1 Catalogued correspondence 2 A-AB 3 AC - ADAMS, J. 4 ADAMS, K.-AG 5 AH-AI 6 AJ-ALD 7 ALE-ALLEN, E. 8 ALLEN, F.-ALLEN, W. 9 ALLEN, Y. - AMERICAN AC. 10 AMERICAN AR. - AMERICAN K. 11 AMERICAN L.-AMZ 12 ANA-ANG 13 ANH-APZ 14 AR-ARZ 15 AS-AT 16 AU-AZ 17 B-BAC 18 BAD-BAKER, G. 19 BAKER, H. - BALDWIN 20 BALE-BANG 21 BANH-BARD 22 BARD-BARNES, J. 23 BARNES, N.-BARO 24 BARR-BARS 25 BART-BAT 26 BAU-BEAM 27 BEAN-BED 28 BEE-BELL, D. 29 BELL,E.-BENED 30 BENEF-BENZ 31 BER-BERN 32 BERN-BETT 33 BETTS-BIK 34 BIL-BIR 35 BIS-BLACK, J. 36 BLACK, K.-BLAN 37 BLANK-BLOOD 38 BLOOM-BLOS 39 BLOU-BOD 40 BOE-BOL 41 BON-BOOK 42 BOOK-BOOT 43 BOR-BOT 44 BOU-BOWEN 45 BOWER-BOYD 46 BOYER-BRAL 47 BRAM-BREG 48 BREH-BRIC 49 BRID - BRIT 50 BRIT-BRO 51 BROG-BROOKS 52 BROOKS-BROWN 53 BROWN 54 BROWN-BROWNE 55 BROWNE -BRYA 56 BRYC - BUD 57 BUE-BURD 58 BURE-BURL 59 BURL-BURR 60 BURS-BUTC 61 BUTLER, A. - S. 62 BUTLER, W.-BYZ 63 C-CAI 64 CAL-CAMPA 65 CAMP - CANFIELD, JAMES H. (-1904) 66 CANFIELD, JAMES H. (1905-1910) - CANT 67 CAP-CARNA 68 CARNEGIE (1) 69 CARNEGIE (2) ENDOWMENT 70 CARN-CARR 71 CAR-CASTLE 72 CAT-CATH 73 CATL-CE 74 CH-CHAMB 75 CHAMC - CHAP 76 CHAR-CHEP 77 CHER-CHILD, K. -

Mental Institutions º
- - - -- - - ------ -- - - - -- * - - ºr . º: - º - - - - - * -- º lºv - - MENTAL INSTITUTIONS 1962 A LISTING OF STATE AND COUNTY MENTAL HOSPITALS AND PUBLIC INSTITUTIONS FOR THE MENTALLY RETARDED U. S. DEPARTMENT OF HEALTH, EDUCATION, AND WELFARE Public Health Service PATIENTS IN MENTAL INSTITUTIONS 1962 A LISTING OF STATE AND COUNTY MENTAL HOSPITALS AND PUBLIC INSTITUTIONS FOR THE MENTALLY RETARDED Prepared by: The National Institute of Mental Health - Biometrics Branch Hospital Studies Section Bethesda, Maryland 20014 U. S. DEPARTMENT OF HEALTH, EDUCATION AND WELFARE Public Health Service National Institutes of Health £4 442 A 3.2, /522 Ape & REFERENJ. St. "As, v 4, # *,§ º * * > * * * Public Health Service Publication No. 1143, Listing Washington, D. C. - 1964 LISTING OF STATE AND COUNTY MENTAL HOSPITALS, AND PUBLIC INSTITUTIONS FOR THE MENTALLY RETARDED The purpose of this publication is to provide, by state and type of facility, a listing of state and county mental hospitals and public institutions for the mentally retarded. These facilities have been classified according to their function rather than by the authority under which they operate. This listing contains only those facilities from which the National Institute of Mental Health requested data for the fiscal year 1962. The 1962 data obtained from these facilities may be found in the following publica tions: Patients in Mental Institutions, 1962 Part I (Public Institutions for the Mentally Retarded) and Part II (State and County Mental Hospitals) U. S. Department of Health, Education, and Welfare, Public Health Service, National Institutes of Health, PHS No. 1143. In these publications, basic census data are provided on the move ment of the patient population, the numbers and characteristics of first admissions (for the public institutions for the mentally retarded) and admissions with no prior psychiatric inpatient experience (for the state and county mental hospitals); the number and characteristics of the resident patients; personnel by occupation; and maintenance expenditures. -

Part I Highlights of This Issue
MONDAY, JULY 1 WASHINGTON, D.C. Volume 40 ■ Numb Pages 29531-29700 PART I HIGHLIGHTS OF THIS ISSUE This listing does not affect the legal status of any document published in this issue. Detailed table of contents appears inside. EMPLOYMENT DISCRIMINATION—ARBA prohibits on basis of religion, sex, age or handicap in federally assisted programs.......................................................... 29536 AMERICAN REVOLUTION BICENTENNIAL—ARBA de scribes Bicentennial logo and prescribes regulations July 14, 1975— Pages 29531-29700 for use.............. .................. ...... .............. *...... ......... ..........i 29539 FOOD STAMPS— USDA/FNS requires States to monitor and improve administration of their programs; effec tive 7-1-75........................ 29531 INCOME TAX— 1RS proposes regulations to determine qualification of certain retirement plans; comments by 8-14-75...... 29553 1RS temporary regulations to determine qualification of certain retirement plans............................................... 29535 CORRECTED MEETING— DOD/AIR: Scientific Advisory Board, 7-30 and 7-31-75.. 29558 (Continued inside) PART II: r j NURSING STUDENT LOANS— HEW/HRA list of hospitals qualifying for loan cancellation............. 29631 rem inders (The items in this list were editorially compiled as an aid to F ederal R eg ister users. Inclusion or exclusion from this list has no legal significance. Since this list is intended as a reminder, it does not include effective dates that occur within 14 days of publication.) Rules Going Into Effect Today This list includes only rules that were published in the F ederal R e g ister after October 1, 1972. DOT/CG— W. Palm Beach Canal, Fla., drawbridge operations.. 25004; 6—12—75 List of Public Laws This is a listing of public bills enacted by Congress and approved by the President, together with the law number, the date of approval, and the U.S. -

Hospital Care at Second Avenue and East 17Th Street, New York City, 1894-1984 Henry Pinsker, M.D., David M
905 HOSPITAL CARE AT SECOND AVENUE AND EAST 17TH STREET, NEW YORK CITY, 1894-1984 HENRY PINSKER, M.D., DAVID M. NovICK, M.D., AND BEVERLY L. RICHMAN, M.D. Beth Israel Medical Center The Mount Sinai School of Medicine of the City University of New York New York, New York B ETH Israel Medical Center's recent sale of the building that housed its Morris J. Bernstein Institute brings to an end nearly 90 years of medi- cal care at Second Avenue and East 17th Street in New York City. During this time, three separate institutions provided care to differing patient popu- lations in the handsome building at 307 Second Avenue. New York Lying- In Hospital, Manhattan General Hospital, and the Morris J. Bernstein In- stitute each contributed to medical progress in areas that at one time received little attention from the medical community. Except for the removal of two ironwork balconies that were found to be unsafe in 1979 and removal of the glass solarium from the roof in 1981, the exterior of the building is substantially as it was when opened in 1902 (Figure 1). A historical perspective of the three hospitals follows. NEW YORK LYING-IN HOPSITAL The New York Lying-In Hospital had its origins in the yellow fever epi- demic of 1798.1 Dr. David Hosack, a prominent practitioner in New York City, observed that many expectant mothers, widowed by the yellow fever, "were rendered wretched under the accumulated evils of grief and poverty." At that time there were no provisions for the medical care of women dur- ing pregnancy or confinement. -

Hospital Musi[ Newsletter
HOSPITAL MUSI[ NEWSLETTER Published by the [ommittee on Music in Hospitals of the National Music [ouncil Ray Green, Editor and Acting Chairman 11 East 10th Street, New York, N . Y. Volume 1 May, 1948 Number 1 Introducing the NEWSLETTER information on special projects established for purposes We hope the few items of information which are in- of exploring the use of hospital music or music research; cluded in this first issue of the HOSPITAL MUSIC announcements of hospital music clinics and training NEWSLETTER will be of gener:!l !.'1terest tc the reader. seminars; other information of a general or specialized Volume 1, No. 1, is a trial balloon that we hope will interest that may be found useful in a hospital music stimulate interest in future issues. We want and invite program. Additional features and sections of the NEWS- your comment and interest. The purpose of the NEWS- LETTER may be added as a need for them arises a.c.d LETTER is the exchange of information between hospi- mterest in them develops. Suggestions from contributors tals on their use of music with patients. We hope that and readers are invited. the pages of the NEWSLETTER will be used frequently by hospitals for this purpose. The Executive Committee of the National Music Exchange of lnformt1tion Council established the National Music Council Music in Hospitals Committee as a means of voluntary co- Hospitals of all types are invited to ordination of the cooperative efforts of member organ- participate in the exchange of information izations in hospital music programs throughout the coun- through the medium of the HOSPITAL try. -

The Hopi Visit Us
.GATHOtl-E Subscription1 :Vol. XXV" No. ·10 June, 1959 25o Per Year Price le • ' STRIKE IN NEW YORK HOSPITALS • At a meeting of the Central Labor Council last week at Roosevelt School Auditorium strikers from each of t he slx hospitals described By DOROTHY DAY the intolerable conditions and low wages of from $32 to $40 a week. Afterwards Beth Israel Hospital was picketed by thousands who The last issue of the Catholic Worker, 'the April-May issue, came marched down 14th. St. from the meeting, out while Deane Mowrer and I The wealthy men who determine the policies of exploitation of the were in the Woman's House of De non-professional employees who are on strike say the same thing now tention. She eovers the story in that they and their kind said years ago, that unless there was a 16 this issue of the paper, and I shall hour day in the steel mills the steel companies would go bankrupt. write more on it later. Looking, In the industrie.s in which they lead they recognize unions_ and pay over my diary in which there are decent wages-because they have to. And because the law states that large gaps I find that part of the non-profit hospitals do not have to bargai.a with unions they take thil time since my last On Pilgrimage, to mean that they can get by with their robbery. was taken up by sickness, an at Lorenzo Santiago of Mt. Sinai H;ospital told of Hungarian refugees tack of flu, the kind that leaves being maqe straw bosses and advised that if they joined the union they one very melancholy, dull-witted would be deported back to Hungary. -

Clinical Thermometers (Third Edition)
CJ lF ‘rs 5 ^ 1 * I i soruf - .' ,' h^’. V 4. V AUG i 7 , ., _ ^ CSl-42 Thermometers* Clinical yi.ui^wii u Vi It- i.iit? Ljijjrgfy . " " U. S. DEPARTMENT OF COMMERCE JESSE Ho JONES, Secretary <2r*fV- ‘»viv„ ! NATIONAL BUREAU OF STANDARDS / ^ LYMAN J. BRIGGS, Director CLINICAL THERMOMETERS (THIRD EDITION) COMMERCIAL STANDARD CSl-42 Effective Date for New Production From February 20, 1942 A RECORDED VOLUNTARY STANDARD OF THE TRADE *0 ® ^ " ' j j J 0 , J . UNITED STATES -) ^ . J J GOVERNMENT PRINTING OFFICE^ WASHINGTON : 1942 ^ A s J * > i ^ • ^ X* 3 j J 3 > 1' " - 1 ^ Tl ii- 1 n - ^ J J J For sale by the Superintendent of Documents, Washington, D. C. - - - - Price 10 cents > 1 > J * National Bureau ot Standards SEP 3 1947 kites' U. S. Department of Commerce National Bureau of Standards PROMULGATION of COMMERCIAL STANDARD CSl-42 for CLINICAL THERMOMETERS (Third Edition) On March 30, 1928, a general conference of representative manu- facturers, distributors, users, and representatives of laboratories adopted a recommended commercial standard for clinical thermome- ters which was accepted by the industry and published as Commercial Standard CSl-28. A revision recommended by the Standing Committee and accepted by the trade was issued in 1932, designated CSl-32. On October 17, 1941, a second revision recommended by the Stand- ing Committee was circulated for written acceptance; those con- cerned have since accepted this revision and approved for promulga- tion by the United States Department of Commerce, through the National Bureau of Standards, the revised standard as shown herein. The standard is effective for new production from February 20, 1942. -
Report Resumes
REPORT RESUMES ED 012 983 'EC 000 244 MENTAL HEALTH DIRECTORY, 1966. BY- YOLLES, STANLEY F. AND OTHERS PUBLIC HEALTH SERVICE, BETHESDA, MD. REPORT NUMBER PI-IS-PUB-1517 PUB DATE 66 FORS PRICE MF-$1.00HC-$8.88 222F. DESCRIPTORS- *DIRECTORIES, *MENTAL HEALTH PROGRAMS, MENTAL HEALTH, NATIONAL CLEARINGHOUSE FOR MENTAL HEALTH INFORMATION THE DIRECTORY IS INTENDED AS A,REFERENCE GUIDE TO MENTAL HEALTH PROGRAMS AND SERVICES THROUGHOUT THE UNITED STATES. IT IS ORGANIZED INTO A FEDERAL SECTION AND A STATE AND COMMUNITY SECTION, EACH OF WHICH IS PRECEDED BY AN INTRODUCTORY STATEMENT CONCERNING THE LISTINGS IN THAT SECTION. ADDRESSES AND SHORT DESCRIPTIONS OF THE MAJOR MENTAL HEALTH PROGRAMS ARE GIVEN FOR OVER 2,000 OUTPATIENT PSYCHIATRIC CLINICS ANC DAY-NIGHT SERVICES IN EACH OF THE STATES. LISTINGS ARE ALPHABETICAL BY STATE, BY CITIES WITHIN THE STATES, AND BY , FACILITIES. PRIVATE MENTAL HOSPITALS, VETERANS ADMINISTRATION HOSPITALS, AND GENERAL HOSPITALS WITH PSYCHIATRIC SERVICES ARE NOT INCLUDED IN THE DIRECTORY. IN ADDITION, THERE IS A LISTING OF MENTAL HEALTH ASSOCIATIONS AND OF OTHER SOURCES OF MENTAL HEALTH INFORMATION. THIS DOCUMENT WAS PUBLISHED BY THE U.S. GOVERNMENT PRINTING OFFICE, WASHINGTON, D.C. $0.60. (RS) . %. NATIONAL CLEARINGHOUSE FOR MENTAL HEALTH INFORMATION 1 -1, w r. 4 , ..-,:;'- U.S..IDEPARTMENVOF___ HEALTH, k5UCA_ -_,' ii. f-ELFARE Public Health Service P .r- 4 The National Clearinghouse for Mental Healthinformation is thescientific and professional informationcenter of the NationalInstitute of MentalHealth - a - DNAL CLEARINGHOUSE FOR MENTAL HEALTH INFORMATION Mental Health Directory 1966 Includes National Institute of Mental Health State Departments Dealing with Mental Health and Mental Retardation State Hospitals for the Mentally Ill and Mentally Retarded Outpatient Psychiatric Clinics and Day-Night Services Mental Health Associations and Other Sources of Mental Health Information U.S. -

Where to Find Medical Records for Closed Hospitals in New York State
Where to Find Medical Records for Closed Hospitals in New York State This document is a list of the last known contacts for storage of hospital medical records in New York State. While every attempt is made to update this list, records may have been relocated, discarded or destroyed in accordance with retention requirements. Note that if there is no location information listed, the Department does not have any information available regarding the location of the records for the facility. Updates to the information on this list may be submitted by selecting “General Inquiries” from the Subject drop down box in the following link: https://apps.health.ny.gov/surveyd8/email-hospdtc#no-back - For information about locating records from psychiatric hospitals, please see the FAQs from the NYS Office of Mental Health at the following link: https://www.omh.ny.gov/omhweb/faq/ - For information on closed hospitals in New York City the following links may be helpful: - https://en.wikipedia.org/wiki/List_of_hospitals_in_New_York_City - http://newyork.resiliencesystem.org/sites/default/files/closed_hospitals.txt Medical Records for Closed Private Practitioners For information about locating medical records from closed private practitioners, you may contact the Office of Professional Medical Conduct at the following link: https://www.health.ny.gov/professionals/doctors/conduct/ https://www.health.ny.gov/professionals/doctors/conduct/contact.htm New York State Education Law 6530.32, available at the link below, establishes the requirements for retention of records: https://www.health.ny.gov/professionals/office-based_surgery/law/6530.htm Unless otherwise provided by law, all patient records must be retained for at least six years. -

Altman Foundation Grants List
Altman Foundation Grants List by year first funded (as of july 2013) legend * Organization was an Altman grantee at some point prior to the 1985 sale of B. Altman & Co. and has also been a grantee in the years since that transition for the Foundation. ° Organization has received Altman grant(s) totaling $1 million or more. 1914 Stadium University Fund Equity Players, Inc. Hebrew Orphan Asylum United Bldg. Fund Campaign of Federated Jewish French and Polyclinic Medical School and Health Hebrew Technical School for Girls Institutions Center Home for Aged and Infirm Hebrews United Jewish Appeal-Federation of Jewish Girls Service League of America Lebanon Hospital /Bronx-Lebanon Hospital Philanthropies of New York, Inc.*° Goldman Concerts Band Center* The Young Women’s Christian Association of the Hartman-Homecrest Library Bureau City of New York* Jewish Education Assn. Montefiore Home/Montefiore Medical Center*° Josephine Home, Inc. St. Mark’s Hospital 1920 Maternity Center Association Cardinal’s Committee of the Laity/Archdiocese of The Metropolitan Museum of Art*° 1917 New York*° The National Stage Woman’s Exchange Allies Bazaar Catholic Charities Archdiocese of New York*° Neighborhood Circle American Red Cross in Greater New York* Flower Hospital New York American Christmas Relief Fund, Inc. Community Service Society of New York* Girl Scout Council of Greater New York, Inc.* The New York Friends of Boys Greater New York Council, Boy Scouts of America Joint Distribution Committee Greater New York New York Guild for Jewish Blind Jewish War Relief Committee—New York Fund New York Heart Association Special Benevolent Society National Urban League, Inc.* The New York Society for the Prevention of Cruelty St.