Capacitive-Coupling Impedance Spectroscopy Using a Non-Sinusoidal Oscillator and Discrete-Time Fourier Transform: an Introductory Study
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Design of Contactless Capacitive Power Transfer Systems for Battery Charging Applications
Design of Contactless Capacitive Power Transfer Systems for Battery Charging Applications By DEEPAK ROZARIO A Thesis Submitted in Partial Fulfilment of the Requirements for the Degree of Master of Applied Science in The Faculty of Engineering and Applied Sciences Program UNIVERSITY OF ONTARIO INSTITUTE OF TECHNOLOGY APRIL, 2016 ©DEEPAK ROZARIO, 2016 ABSTRACT Several forms for wireless power transfer exists - Microwave, Laser, Sound, Inductive, Capacitive etc. Among these, the Inductive Power Transfer Systems (IPT) are the most extensively used form of wireless power transfer. Due to the utilization of magnetics the inductive power transfer system suffers from Electromagnetic Interference (EMI) issues. Due to the utilization of magnetic field to transfer power, the system is not preferred in an environment with metals and cannot transfer power through metal barriers. Capacitive Power Transfer System (CPT) is an emerging field in the area of wireless power transfer. The antennae of the CPT system, constitute two metal plates which are separated by a dielectric (air). When energised, the metal plates along with the dielectric resemble a loosely coupled capacitor, hence the term capacitive power transfer. The capacitive system utilizes electric field to transfer power and therefore eliminating electromagnetic interference issues. The system has low standing power losses, good anti-interference ability. The advantages, make the CPT system a dynamic alternative to the traditional wireless inductive system. As the area is still in its infancy, the first part of this thesis is dedicated to an extensive study on the literature available on the CPT systems and the basic operation of the system. From, the study it was evident that CPT systems have efficiencies ranging between 60% to 80%. -

Operational Amplifier “Op Amp”
Buffering • You saw that the parallel resistor lowers the voltage • A voltage measurement device with a non-infinite resistance does the same; we would therefore like a way to connect a voltmeter to the touchscreen without loading the system and lowering the voltage • This is easily done using a buffer. A buffer has a high input resistance, but can source the current needed by the load. Energy needed by load from another supply as needed. Input voltage High input reproduced at resistance output • In effect, a buffer (nearly) reproduces the input voltage, but doesn’t load the input • Note that a buffer cannot produce energy, so it draws the energy the load requests from some other power supply 49 Amplifier Integrated Circuits • In an ideal world, an amplifier IC takes an input signal (for example, Vin), and multiplies it by a fixed amount to produce an output signal. Example: Vout = AVVin where AV is the multiplier, called a voltage gain • Of course, the energy for this multiplication has to come from somewhere. Therefore, an amplifier IC has power supply connections as well. 50 Operational Amplifier “Op Amp” • Two input terminals, positive (non- inverting) and negative (inverting) • One output • Power supply V+ , and Op Amp with power supply not shown (which is how we usually display op amp circuits) 51 Equivalent Circuit and Specifications • In other words, a really good buffer, since Ri . All the needed power for the output is drawn from the supply 52 Gain of an Op Amp • Key characteristic of op amp: high voltage gain • Output, A, is -

Analysis and Modeling of Capacitive Coupling Along Metal Interconnect Lines by Andrew K
Analysis and Modeling of Capacitive Coupling Along Metal Interconnect Lines by Andrew K. Percey Submitted to the Department of Electrical Engineering and Computer Science in partial fulfillment of the requirements for the degrees of Bachelor of Science in Electrical Science and Engineering and Master of Engineering in Electrical Engineering and Computer Science at the MASSACHUSETTS INSTITUTE OF TECHNOLOGY June 1996 @ Andrew K. Percey, MCMXCVI. All rights reserved. The author hereby grants to MIT permission to reproduce and distribute publicly paper and electronic copies of this thesis document in whole or in part, and to grant others the right to do so. MASSACH'USETTS NSTi'U L' OF TECHNOLOGY JUN 1 1996 Author............ LIBRARIES Department of Electrical Engineering andCo mputer Science Eng. May 28, 1996 Certified by .......... ........ ...... Jacob White Professor of Electrical Engineering ",sis Supervisor A ccepted ',, ....I........... .. ............... ............. F. R. Morgenthaler Chairman, Departme Committee on Graduate Theses Analysis and Modeling of Capacitive Coupling Along Metal Interconnect Lines by Andrew K. Percey Submitted to the Department of Electrical Engineering and Computer Science on May 28, 1996, in partial fulfillment of the requirements for the degrees of Bachelor of Science in Electrical Science and Engineering and Master of Engineering in Electrical Engineering and Computer Science Abstract Electrical signals propagating along metal interconnect lines within contemporary microchips experience significant delay and noise due to capacitive coupling effects. Analysis and modeling of these effects was performed at the author's VI-A Internship company. Numerous CAD circuit simulations were performed to acquire a better understanding of these coupling effects. A program was created to assist circuit designers in analyzing these effects for their particular circuit topologies. -

Noise Coupling Models in Heterogeneous 3-D Ics Boris Vaisband, Student Member, IEEE, and Eby G
2778 IEEE TRANSACTIONS ON VERY LARGE SCALE INTEGRATION (VLSI) SYSTEMS, VOL. 24, NO. 8, AUGUST 2016 Noise Coupling Models in Heterogeneous 3-D ICs Boris Vaisband, Student Member, IEEE, and Eby G. Friedman, Fellow, IEEE Abstract— Models of coupling noise from an aggressor module to a victim module by way of through silicon vias (TSVs) within heterogeneous 3-D integrated circuits (ICs) are presented in this paper. Existing TSV models are enhanced for different substrate materials within heterogeneous 3-D ICs. Each model is adapted to each substrate material according to the local noise coupling characteristics. The 3-D noise coupling system is evaluated for isolation efficiency over frequencies of up to 100 GHz. Isolation improvement techniques, such as reducing the ground network inductance and increasing the distance between the aggressor and victim modules, are quantified in terms of noise improvements. A maximum improvement of 73.5 dB for different ground network impedances and a difference of 38.5 dB in isolation efficiency for greater separation between the aggressor and victim modules are demonstrated. Compact, accurate, and computationally efficient models are extracted from the transfer function for each of the heterogeneous substrate materials. The reduced transfer functions are used to explore different manufacturing and design parameters to evaluate coupling noise across multiple 3-D planes. Index Terms— 3-D integrated circuit (IC), heterogeneous 3-D system, noise coupling, substrate coupling, through silicon via (TSV) noise coupling model. Fig. 1. Heterogeneous 3-D IC. I. INTRODUCTION OISE coupling is of increasing importance within the TABLE I integrated circuits (ICs) community [1]–[6]. -

AN-937 Designing Amplifier Circuits
AN-937 APPLICATION NOTE One Technology Way • P. O. Box 9106 • Norwood, MA 02062-9106, U.S.A. • Tel: 781.329.4700 • Fax: 781.461.3113 • www.analog.com Designing Amplifier Circuits: How to Avoid Common Problems by Charles Kitchin INTRODUCTION down toward the negative supply. The bias voltage is amplified When compared to assemblies of discrete semiconductors, by the closed-loop dc gain of the amplifier. modern operational amplifiers (op amps) and instrumenta- This process can be lengthy. For example, an amplifier with a tion amplifiers (in-amps) provide great benefits to designers. field effect transistor (FET) input, having a 1 pA bias current, Although there are many published articles on circuit coupled via a 0.1-μF capacitor, has an IC charging rate, I/C, of applications, all too often, in the haste to assemble a circuit, 10–12/10–7 = 10 μV per sec basic issues are overlooked leading to a circuit that does not function as expected. This application note discusses the most or 600 μV per minute. If the gain is 100, the output drifts at common design problems and offers practical solutions. 0.06 V per minute. Therefore, a casual lab test, using an ac- coupled scope, may not detect this problem, and the circuit MISSING DC BIAS CURRENT RETURN PATH may not fail until hours later. It is important to avoid this One of the most common application problems encountered is problem altogether. the failure to provide a dc return path for bias current in ac- +VS coupled op amp or in-amp circuits. -

Harmonic Distortion in Renewable Energy Systems: Capacitive Couplings
11 Harmonic Distortion in Renewable Energy Systems: Capacitive Couplings Miguel García-Gracia, Nabil El Halabi, Adrián Alonso and M.Paz Comech CIRCE (Centre of Research for Energy Resources and Consumption) University of Zaragoza Spain 1. Introduction Renewable energy systems such as wind farms and solar photovoltaic (PV) installations are being considered as a promising generation sources to cover the continuous augment demand of energy. With the incoming high penetration of distributed generation (DG), both electric utilities and end users of electric power are becoming increasingly concerned about the quality of electric network (Dugan et al., 2002). This latter issue is an umbrella concept for a multitude of individual types of power system disturbances. A particular issue that falls under this umbrella is the capacitive coupling with grounding systems, which become significant because of the high-frequency current imposed by power converters. The major reasons for being concerned about capacitive couplings are: a. Increase the harmonics and, thus, power (converters) losses in both utility and customer equipment. b. Ground capacitive currents may cause malfunctioning of sensitive load and control devices. c. The circulation of capacitive currents through power equipments can provoke a reduction of their lifetime and limits the power capability. d. Ground potential rise due to capacitive ground currents can represent unsafe conditions for working along the installation or electric network. e. Electromagnetic interference in communication systems and metering infrastructure. For these reasons, it has been noticed the importance of modelling renewable energy installations considering capacitive coupling with the grounding system and thereby accurately simulate the DC and AC components of the current waveform measured in the electric network. -
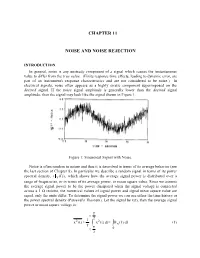
Chapter 11 Noise and Noise Rejection
CHAPTER 11 NOISE AND NOISE REJECTION INTRODUCTION In general, noise is any unsteady component of a signal which causes the instantaneous value to differ from the true value. (Finite response time effects, leading to dynamic error, are part of an instrument's response characteristics and are not considered to be noise.) In electrical signals, noise often appears as a highly erratic component superimposed on the desired signal. If the noise signal amplitude is generally lower than the desired signal amplitude, then the signal may look like the signal shown in Figure 1. Figure 1: Sinusoidal Signal with Noise. Noise is often random in nature and thus it is described in terms of its average behavior (see the last section of Chapter 8). In particular we describe a random signal in terms of its power spectral density, (x (f )) , which shows how the average signal power is distributed over a range of frequencies, or in terms of its average power, or mean square value. Since we assume the average signal power to be the power dissipated when the signal voltage is connected across a 1 Ω resistor, the numerical values of signal power and signal mean square value are equal, only the units differ. To determine the signal power we can use either the time history or the power spectral density (Parseval's Theorem). Let the signal be x(t), then the average signal power or mean square voltage is: T t 2 221 x(t) x(t)dtx (f)df (1) T T t 0 2 11-2 Note: the bar notation, , denotes a time average taken over many oscillations of the signal. -

2. Capacitors Contents
2. Capacitors Contents 1 Capacitor 1 1.1 History ................................................. 2 1.2 Theory of operation .......................................... 2 1.2.1 Overview ........................................... 3 1.2.2 Hydraulic analogy ....................................... 3 1.2.3 Energy of electric field .................................... 4 1.2.4 Current–voltage relation ................................... 4 1.2.5 DC circuits .......................................... 4 1.2.6 AC circuits .......................................... 5 1.2.7 Laplace circuit analysis (s-domain) .............................. 5 1.2.8 Parallel-plate model ...................................... 5 1.2.9 Networks ........................................... 6 1.3 Non-ideal behavior .......................................... 7 1.3.1 Breakdown voltage ...................................... 7 1.3.2 Equivalent circuit ....................................... 7 1.3.3 Q factor ............................................ 8 1.3.4 Ripple current ......................................... 8 1.3.5 Capacitance instability .................................... 8 1.3.6 Current and voltage reversal ................................. 9 1.3.7 Dielectric absorption ..................................... 9 1.3.8 Leakage ............................................ 9 1.3.9 Electrolytic failure from disuse ................................ 9 1.4 Capacitor types ............................................ 9 1.4.1 Dielectric materials ..................................... -

Capacitive Coupling Inductive 3
NHP Electrical Engineering Products Pty Ltd A.B.N. 84 004 304 812 www.nhp.com.au AUSTRALIA 100 percent Australian Owned VICTORIA MELBOURNE 43-67 River Street 5 6 Richmond VIC 3121 Phone (03) 9429 2999 are at the closest (see not all solutions can be shielding. It is definitely with the o Fax (03) 9429 1075 recommend- ideal angle is at 90 . NEW SOUTH WALES equation.C: (cos ø = found and explained. earthing that most ECHNICAL NEWS SYDNEY T cos90=0 ). However, most problems Filtering problems occur. There ations F) Optic fibre cables are 30-34 Day Street North, Issue 29 October 1999 can be solved by is a major difference increasingly attractive Silverwater NSW 2128 Phone (02) 9748 3444 observing the following between a safety earth alternatives to copper Please circulate to Shielding for The use of filters in A) The communications Fax (02) 9648 4353 guidelines. and a EMI earth, cables should be laid as conductors for data NEWCASTLE _________________________ suppressing EMI from communications circuits 575 Maitland Road inductive especially at higher far from the power Mayfield West NSW 2304 _________________________ The main problem of EMI VSDs is aimed at Quarterly Technical frequencies. What is cables as possible at the in high interference Phone (02) 4960 2220 _________________________ is interference to sensitive preventing the Fax (02) 4960 2203 Newsletter of Australia’s coupling needed for EMI is a low outer extremes of the environments. Fibre _________________________ equipment nearby. Noise interference passing QUEENSLAND leading supplier of EMC- What’s HF impedance earth cable ladder or duct. cables do not suffer from BRISBANE low-voltage motor _________________________ is coupled into the down the lines and also Surrounding the victim system. -

Noise Sources in Applications Using Capacitive Coupled Isolated Amplifiers
®APPLICATION BULLETIN Mailing Address: PO Box 11400 • Tucson, AZ 85734 • Street Address: 6730 S. Tucson Blvd. • Tucson, AZ 85706 Tel: (602) 746-1111 • Twx: 910-952-111 • Telex: 066-6491 • FAX (602) 889-1510 • Immediate Product Info: (800) 548-6132 NOISE SOURCES IN APPLICATIONS USING CAPACITIVE COUPLED ISOLATED AMPLIFIERS By Bonnie C. Baker (602) 746-7984 Noise is a typical problem confronting many isolation appli- duce distortion. As shown in Figure 1, there are three cations. Isolation products such as analog isolation amplifi- primary types of noise endemic to isolation applications, ers, optocouplers, transformers and digital couplers, are used each with their own set of possible solutions. The first noise in applications to transmit signals across a high voltage source is device noise. Device noise is the intrinsic noise of barrier while providing galvanic separation between two the devices in the circuit. Examples of device noise would be grounds. Burr-Brown’s isolated analog amplifiers and digi- the thermal noise of a resistor or the shot noise of a tal couplers use one of three coupling technologies in their transistor. A second source of noise that effects the perfor- isolation products, each having its own set of advantages and mance of isolation devices is conductive noise. This type of disadvantages in noisy environments. These technologies noise already exists in the conductive paths of the circuit, are inductive coupling, capacitive coupling and optical cou- such as the power lines, and mixes with the desired electrical pling. Isolation amplifiers and digital couplers are used for signal through the isolation device. The third source of noise a variety of applications including breaking of ground loops, is radiated noise. -

Effect of Surrounding Conductive Object on Four-Plate Capacitive
Effect of Surrounding Conductive Object on Four- Plate Capacitive Power Transfer System Qi Zhu, Lixiang Jackie Zou, Shaoge Zang, Mei Su, and Aiguo Patrick Hu Abstract- In this paper, the effect of a surrounding conductive in the secondary side as power receivers. The typical structure object on a typical capacitive power transfer (CPT) system with of a four-plate CPT system is shown in Fig. 1. two pairs of parallel plates is studied by considering the mutual Based on the above typical structure, some fundamental coupling between the conductive object and the plates. A studies have been done. C. Liu et al. studied the coupling mathematical model is established based on a 5*5 mutual mechanism [15], steady-state analysis [16], power flow control capacitance matrix by using a larger additional conductive plate to represent the surrounding conductive object. Based on the [17], 2D alignment analysis [18] and generalized coupling proposed model, the effect of the additional conductive plate on modeling [19] of a four-plate CPT system. L. Huang et al. the CPT system is analyzed in detail. The electric field distribution studied compensation networks for improving the performance of the CPT system including the additional plate is simulated by of a four-plate CPT system [20-21], the accurate steady-state ANSYS Maxwell. A practical CPT system consisting of four modeling considering cross-coupling for a four-plate CPT 100mm*100mm square aluminum plates and one 300mm*300mm system [22] and the definition of mutual coupling among four square aluminum plate is built to verify the modeling and analysis. -

Terminology Pocket Guide
4 Cap Array: A/D: AC: Accu L: Accu F: Accu P: AccuGuard: Act ve Components: Alternating Current: Aluminum Electrolytic: Ambient Noise: Ambient Temperature: Ammo-Pack: Ampere: Analog Circuit: Anode: Automatic Insertion: Axial Leads: B Tolerance: Backpanel: Balun: Bandpass Filter: Bandwidth: Battery: Baud Rate: Baud: BitGuard: Blocking Capacitor: Board to Board: Bond: Boost Capacitor: Boxed Film (BF): BPS: Breadboard: Breakdown Voltage: Bulk Cassette: Bulk Packaging: Bulkhead Filter: Burn-in: Bypass Capacitor: C Tolerance: C: C0G/NP0: Capacitance: Capacitive Coupling: Capacitor: CapGuard: Cathode: CDR: Celsius: Centerline: Ceramic Package: Chip: CK: CKR: Clean-Room: Clock Oscillator: Clock Rate: Coaxial Cable: Cofired Ceramic: Coil: Conductor: Conformal Coating: Contact: CPU: Crystal: Current: CV: Cycle: D Tolerance: D/A: Damping: Date Code: DC: Decibel (db): Decoupling: Die: Dielectric Constant: Dielectric Strength: Dielectric: Digital Circuit: DIN41612: Diode: DipGuard: Dipped: Direct Current (DC): Disk/Disc: Dissipation: Dual-in-Line: E12: E24: E6: ECOAX: ECOCAP: ECOMP 1,2,3,4: EIA: Electrode: Electrolyte: Electrolytic Capacitor: Electrostatic: Encapsulate: Energy Density: ESL: ESR: Extended Range: F Tolerance: Fahrenheit: Failure Analysis: Farad: Feedthrough: Ferrite: FFC/FPC: Film Capacitor: Film: Filter (LPF/BPF): Fire: Flip-chip: Flyback Transformer (FPT): FM: Frequency: G Tolerance: GHz: Gigahertz (GHz): Glass Capacitor: Glass K: Ground: Harmonic: Henry (µH): Hermetic-sealing: Hertz: High Frequency: High Q: Hybrid: Hz: IDCapacitor: