Heptachlor for Possible Carcinogenicity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A COMPARATIVE STUDY of the CYTOGENETIC EFFECTS of the INSECTICIDES HEPTACHLOR, MALATHION, and METHYL PARATHION in Vicia Faba
Contam. Amb. 1, 7-16, 1985 A COMPARATIVE STUDY OF THE CYTOGENETIC EFFECTS OF THE INSECTICIDES HEPTACHLOR, MALATHION, AND METHYL PARATHION IN Vicia faba SANDRAGOMEZ-ARROYO, ANA MAR~ABAIZA, GRA~IELALOPEZ AND RAFAELVILLALOBOS- PIETRINI Laboratorio de Citogenética y Mutagénesis Am- bientales, Centro de Ciencias de la Atmósfera, Universidad Nacional Autónoma de México, Co- yoacán 04510, México, D. F. y Centro de Inves- tigación y Reproducción Animal, Universidad Au- tónoma de Tlaxcala. ABSTRACT In order to determine the potential cytogenetic effects of Heptachlor, Malathion and Methyl Parathion, meristems of broad bean root tips were treated with se- vera1 concentrations o£ these insecticides for different periods of treatment and recovery. Heptachlor and Malathion induced chromosomal alterations in anaphase cells as fragments and bridges, chromosomes with inactivated centromeres and isochroinosomes, and as micronuclei in interphase cells. Multipolar anaphases also appeared when the mitotic spindle was damaged. Heptachlor induced pycnosis but Malathion did not. Chromosomal aberrations caused by Methyl Parathion were observed in cells only during metaphase, since a strong c-mitotic effect was induced mostly in the form of fragments. Longer recovery periods (42 and 44 h) revealed tetraploid and pycnotic cells. Only in the case of Malation was there an increase in chromosome altera- tions with higher concentrations. However, no such dose response relationship was observed for micronuclei frequency. RESUMEN Con el fin de establecer el daño genético que ocasionan el Heptacloro, el Mala- tión y el Metil Paratión, se realizaron tratamientos sobre las células meristemá- ticas de la raíz de haba con diversas concentraciones de estos insecticidas y dife- rentes tiempos de tratamiento y recuperación. -

TOXICOLOGICAL PROFILE for HEPTACHLOR and HEPTACHLOR EPOXIDE
TOXICOLOGICAL PROFILE FOR HEPTACHLOR and HEPTACHLOR EPOXIDE Prepared by: Syracuse Research Corporation Under Contract No. 200-2004-09793 Prepared for: U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service Agency for Toxic Substances and Disease Registry November 2007 HEPTACHLOR AND HEPTACHLOR EPOXIDE ii DISCLAIMER The use of company or product name(s) is for identification only and does not imply endorsement by the Agency for Toxic Substances and Disease Registry. HEPTACHLOR AND HEPTACHLOR EPOXIDE iii UPDATE STATEMENT A Toxicological Profile for Heptachlor/Heptachlor Epoxide, Draft for Public Comment was released in September 2005. This edition supersedes any previously released draft or final profile. Toxicological profiles are revised and republished as necessary. For information regarding the update status of previously released profiles, contact ATSDR at: Agency for Toxic Substances and Disease Registry Division of Toxicology and Environmental Medicine/Applied Toxicology Branch 1600 Clifton Road NE Mailstop F-32 Atlanta, Georgia 30333 HEPTACHLOR AND HEPTACHLOR EPOXIDE iv This page is intentionally blank. v FOREWORD This toxicological profile is prepared in accordance with guidelines developed by the Agency for Toxic Substances and Disease Registry (ATSDR) and the Environmental Protection Agency (EPA). The original guidelines were published in the Federal Register on April 17, 1987. Each profile will be revised and republished as necessary. The ATSDR toxicological profile succinctly characterizes the toxicologic and adverse health effects information for the hazardous substance described therein. Each peer-reviewed profile identifies and reviews the key literature that describes a hazardous substance's toxicologic properties. Other pertinent literature is also presented, but is described in less detail than the key studies. -

Pesticide Residue Monitoring in Sediment and Surface Water Within the South Florida Water Management District Volume 2
Technical Publication 91-01 Pesticide Residue Monitoring in Sediment and Surface Water Within the South Florida Water Management District Volume 2 by Richard J. Pfeuffer January 1991 This publication was produced at an annual cost of $243.75 or $.49 per copy to inform the public. 500 191 Produced on recycled paper. Water Quality Division Research and Evaluation Department South Florida Water Management District West Palm Beach, Florida A IBSTRAC'I' Pesticide monitoring data are collected under the requirements of several permits and agreements as an indicator of water quality. The monitoring provides data to determine shifts or adverse trends in the quality of water being delivered to Lake Okeechobee, Everglades National Park, and the Water Conservation Areas. In addition, pesticide residue data are collected throughout the South Florida Water Management District at locations selected to determine water quality conditions at the major water control points. Special investigations are performed on selected pesticides as required and follow-up sampling is conducted based on the pesticides detected. Data were collected from 13 stations in 1984. By 1988, the network was expanded to 29 stations. Currently, water and sediment samples are collected quarterly and analyzed for 67 pesticides, herbicides and degradation products. Out of a total of 197 surface water samples, 13 percent had detectable residues, while 25 percent of the 208 sediment samples had detectable residues. The compounds detected in the water samples included atrazine and zinc phosphide while a variety of compounds, including DDT, have been detected in the sediment. None of the residues detected are considered to have adverse health or environmental effects. -

Past Use of Chlordane, Dieldrin, And
The Hazard Evaluation and Emergency Response Office (HEER Office) is part of the Hawai‘i Department of Health (HDOH) Environmental Health Administration, whose mission is to protect human health and the environment. The HEER Office provides leadership, support, and partnership in preventing, planning for, responding to, and enforcing environmental laws relating to releases or threats of releases of hazardous substances. Past Use of Chlordane, Dieldrin, and other Organochlorine Pesticides for Termite Control in Hawai‘i: Safe Management Practices around Treated Foundations or during Building Demolition This fact sheet provides building owners, demolition and construction contractors, developers, realtors, and others with an overview of the potential environmental concerns associated with the past use of organochlorine termiticides (pesticides used to control termites) in Hawai‘i. In addition, this fact sheet discusses methods for reducing exposure to organochlorine termiticides during building demolition or around the foundations of treated buildings and identifies resources for further information. What are organochlorine termiticides? Organochlorine termiticides are a group of pesticides that were used for termite control in and around wooden buildings and homes from the mid-1940s to the late 1980s. These organochlorine pesticides included chlordane, aldrin, dieldrin, heptachlor, and dichlorodiphenyltrichloroethane (DDT). They were used primarily by pest control operators in Hawaii’s urban areas, but also by homeowners, the military, the state, and counties to protect buildings against termite damage. In the 1970s and 1980s, the U.S. Environmental Protection Agency (EPA) banned all uses of these organochlorine pesticides except for heptachlor, which can be used today only for control of fire ants in underground power transformers. -

P-Listed Hazardous Wastes
P-Listed Hazardous Wastes The Environmental Protection Agency (EPA) has identified a number of chemicals on the EPA “P-list” that present an especially acute hazard when disposed of as hazardous waste. Because of their acute hazards, there are more stringent requirements when disposing of these wastes: ►Container size: When collecting p-listed chemicals as waste, the volume of the hazardous waste container must not exceed one quart (approximately one liter). ►Empty containers: Empty containers that held p-listed chemicals must also be disposed of as hazardous waste. They are not allowed to be washed or re-used. ►Contaminated materials: Disposable materials that become contaminated with p-listed chemicals (e.g. gloves, weighing boats, etc.) must also be disposed of as hazardous waste. Non-disposable materials must be “triple-rinsed”, or rinsed three times to remove the contamination. This rinsate must be collected as hazardous waste. Materials contaminated with p-listed chemicals may not be washed or re-used until they have been triple-rinsed. Remember: - Label the waste as hazardous waste. Most common p-listed wastes Just like all other hazardous wastes, p-listed Chemical CAS number wastes must be labeled with the words Acrolein 107–02–8 “hazardous waste”, the complete chemical Allyl alcohol 107–18–6 name, and the associated hazard Arsenic compounds Varies characteristics (e.g., ignitable, corrosive, Inorganic cyanide Varies toxic, or reactive). salts Carbon disulfide 75-15-0 - Use disposable materials whenever Cyanogen and 460-19-5, 506-77-4 possible. Triple-rising non-disposable Cyanogen Chloride material generates a lot of waste, which can 2,4-Dinitrophenol 51–28–5 be difficult to dispose of safely. -

Download in English
UNITED NATIONS BC UNEP/CHW.12/5/Add.9 Distr.: General 10 July 2015 Original: English Conference of the Parties to the Basel Convention on the Control of Transboundary Movements of Hazardous Wastes and Their Disposal Twelfth meeting Geneva, 4–15 May 2015 Agenda item 4 (b) (i) Matters related to the implementation of the Convention: scientific and technical matters: technical guidelines Technical guidelines Technical guidelines on the environmentally sound management of wastes consisting of, containing or contaminated with the pesticides aldrin, alpha hexachlorocyclohexane, beta hexachlorocyclohexane, chlordane, chlordecone, dieldrin, endrin, heptachlor, hexachlorobenzene, lindane, mirex, pentachlorobenzene, perfluorooctane sulfonic acid, technical endosulfan and its related isomers or toxaphene or with hexachlorobenzene as an industrial chemical Note by the Secretariat At its twelfth meeting, the Conference of the Parties to the Basel Convention on the Control of Transboundary Movements of Hazardous Wastes and Their Disposal adopted, in decision BC-12/3 on technical guidelines on the environmentally sound management of wastes consisting of, containing or contaminated with persistent organic pollutants, the technical guidelines on the environmentally sound management of wastes consisting of, containing or contaminated with the pesticides aldrin, alpha hexachlorocyclohexane, beta hexachlorocyclohexane, chlordane, chlordecone, dieldrin, endrin, heptachlor, hexachlorobenzene, lindane, mirex, pentachlorobenzene, perfluorooctane sulfonic acid, technical endosulfan and its related isomers or toxaphene or with hexachlorobenzene as an industrial chemical, on the basis of the draft technical guidelines contained in document UNEP/CHW.12/INF/15. The technical guidelines referred to above were prepared by the Food and Agriculture Organization of the United Nations as lead organization for this work taking into account comments received from members of the small intersesssional working group on persistent organic pollutants wastes by 27 March 2015. -

Code Chemical P026 1-(O-Chlorophenyl)Thiourea P081 1
Code Chemical P026 1-(o-Chlorophenyl)thiourea P081 1,2,3-Propanetriol, trinitrate (R) P042 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]-, (R)- P067 1,2-Propylenimine P185 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)- carbonyl]oxime 1,4,5,8-Dimethanonaphthalene, 1,2,3,4,10,10-hexa- chloro-1,4,4a,5,8,8a,-hexahydro-, P004 (1alpha,4alpha, 4abeta,5alpha,8alpha,8abeta)- 1,4,5,8-Dimethanonaphthalene, 1,2,3,4,10,10-hexa- chloro-1,4,4a,5,8,8a-hexahydro-, P060 (1alpha,4alpha, 4abeta,5beta,8beta,8abeta)- P002 1-Acetyl-2-thiourea P048 2,4-Dinitrophenol P051 2,7:3,6-Dimethanonaphth [2,3-b]oxirene, 3,4,5,6,9,9 -hexachloro-1a,2,2a,3,6,6a,7,7a- octahydro-, (1aalpha,2beta,2abeta,3alpha,6alpha,6abeta,7 beta, 7aalpha)-, & metabolites 2,7:3,6-Dimethanonaphth[2,3-b]oxirene, 3,4,5,6,9,9- hexachloro-1a,2,2a,3,6,6a,7,7a- P037 octahydro-, (1aalpha,2beta,2aalpha,3beta,6beta,6aalpha,7 beta, 7aalpha)- P045 2-Butanone, 3,3-dimethyl-1-(methylthio)-, O-[methylamino)carbonyl] oxime P034 2-Cyclohexyl-4,6-dinitrophenol 2H-1-Benzopyran-2-one, 4-hydroxy-3-(3-oxo-1- phenylbutyl)-, & salts, when present at P001 concentrations greater than 0.3% P069 2-Methyllactonitrile P017 2-Propanone, 1-bromo- P005 2-Propen-1-ol P003 2-Propenal P102 2-Propyn-1-ol P007 3(2H)-Isoxazolone, 5-(aminomethyl)- P027 3-Chloropropionitrile P047 4,6-Dinitro-o-cresol, & salts P059 4,7-Methano-1H-indene, 1,4,5,6,7,8,8-heptachloro- 3a,4,7,7a-tetrahydro- P008 4-Aminopyridine P008 4-Pyridinamine P007 5-(Aminomethyl)-3-isoxazolol 6,9-Methano-2,4,3-benzodioxathiepin, 6,7,8,9,10,10- -

NPL Site Narrative for Chevron Chemical Co. (Ortho Division) NPL Site Listing Process NPL Site Narrative for Chevron Locate NPL Sites Chemical Co
National Priorities List Contact Us | Print Version Search: EPA Home > Superfund > Sites > National Priorities List (NPL) > Locate NPL Sites > NPL Sites in the US > NPL Site Narrative for Chevron Chemical Co. (Ortho Division) NPL Site Listing Process NPL Site Narrative for Chevron Locate NPL Sites Chemical Co. (Ortho Division) NPL Site Status Information CHEVRON CHEMICAL CO. (ORTHO DIVISION) Orlando, Florida Current NPL Updates: New Proposed and Federal Register Notice: May 31, 1994 New Final NPL Sites NPL Resources The Chevron Chemical Co. Inc. (Ortho Division) site is located Basic Query in Orlando, Orange County, Florida. The site covers Advanced Query approximately 4.39 acres in an industrial area at 3100 Orange NPL Sites in the US Blossom Trail. As a result of an EPA removal action, all HRS Toolbox concrete, buildings, and the water tower have been removed. Federal Register The Chevron Chemical Co. operated a chemical blending Notices for facility for pesticides and other crop sprays between 1950 and NPL Updates 1976. The facility formulated a variety of liquid and powdered pesticides, citric sprays, and nutritional sprays. Chemicals used in pesticide formulation included xylene, kerosene, mineral oil, and aromatic naphtha. A few of the pesticides formulated in large volumes consisted of chlordane, lindane, dieldrin, and aldrin. Prior to 1970, two unlined rinsate ponds onsite were used for the collection and disposal of pesticide formulating rinse water, barrel rinse water, and storm water. After 1970, the pesticide formulating rinsate was collected and disposed of offsite at an unknown location. Chevron Chemical ceased operations in 1976. The remaining inventories were removed from the site and the rinsate ponds were backfilled with soil prior to the sale of the property in 1978 to Robert R. -

European Corn Borer Control with Granular Formulations of Endrin, Heptachlor, and Toxaphene Mahlon Lowell Fairchild Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1959 European corn borer control with granular formulations of endrin, heptachlor, and toxaphene Mahlon Lowell Fairchild Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Zoology Commons Recommended Citation Fairchild, Mahlon Lowell, "European corn borer control with granular formulations of endrin, heptachlor, and toxaphene " (1959). Retrospective Theses and Dissertations. 2152. https://lib.dr.iastate.edu/rtd/2152 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. EUROPEAN CORN BORER CONTROL WITH GRANULAR FORMULATIONS OF ENDRIN, HEPTACHLOR, AND TOXAPHENE by MaliIon Lowell Fairchild A Dissertation Submitted to the Graduate Faculty in Partial Fulfillment of The Requirements for the Degree of DOCTOR OF PHILOSOPHY Major Subject: Entomology Approved: Signature was redacted for privacy. Signature was redacted for privacy. Heati of Major department Signature was redacted for privacy. Iowa State Col 1ege Ames, Iowa 1959 I I TABLE OF CONTENTS INTRODUCTION REVIEW OF LITERATURE METHODS AND PROCEDURE DISCUSSION AND RESULTS SUMMARY AND CONCLUSIONS LITERATURE CITED ACKNOWLEDGMENTS APPENDIX ] INTRODUCTION The European corn borer (Pyrausta nubi la 1 is (libn. ) ) was first re ported in this country by VinaI (1917). He suggested that it might have been imported in broomcorn from Europe. Since then it has moved west ward and southward until now it covers a major part of the corn growing area in the United States and Canada and has become one of the major economic pests on corn. -
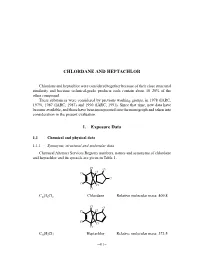
Chlordane and Heptachlor
CHLORDANE AND HEPTACHLOR Chlordane and heptachlor were considered together because of their close structural similarity and because technical-grade products each contain about 10–20% of the other compound. These substances were considered by previous working groups, in 1978 (IARC, 1979), 1987 (IARC, 1987) and 1990 (IARC, 1991). Since that time, new data have become available, and these have been incorporated into the monograph and taken into consideration in the present evaluation. 1. Exposure Data 1.1 Chemical and physical data 1.1.1 Synonyms, structural and molecular data Chemical Abstract Services Registry numbers, names and synonyms of chlordane and heptachlor and its epoxide are given in Table 1. Cl Cl Cl Cl Cl Cl Cl Cl C10H6Cl8 Chlordane Relative molecular mass: 409.8 Cl Cl Cl Cl Cl Cl Cl C10H5Cl7 Heptachlor Relative molecular mass: 373.5 –411– 412 IARC MONOGRAPHS VOLUME 79 Table 1. Chemical Abstract Services Registry numbers, names and synonyms of chlordane, heptachlor and its epoxide Name CAS Reg. Nosa Chem. Abstr. namesb and synonyms Chlordane 57-74-9 ENT 9932; 1,2,4,5,6,7,8,8-octachloro-2,3,3a,4,7,7a- (39400-80-1); hexahydro-4,7-methano-1H-indene; 1,2,4,5,6,7,8,8- 53637-13-1) octachloro-2,3,3a,4,7,7a-hexahydro-4,7-methano- indene (IUPAC); octachloro-4,7-methanotetrahydro- indane; 1,2,4,5,6,7,8,8-octachloro-3a,4,7,7a-tetra- hydro-4,7-methanoindan; OMS 1437 Technical-grade 12789-03-6 chlordane cis-Chlordane 5103-71-9 α-Chlordan; α-chlordane; cis-chlordan; (152322-29-7; (1α,2α,3aα,4β,7β,7aα)-1,2,4,5,6,7,8,8-octachloro- 22212-52-8; -

Chemical Compatibility Storage Group
CHEMICAL SEGREGATION Chemicals are to be segregated into 11 different categories depending on the compatibility of that chemical with other chemicals The Storage Groups are as follows: Group A – Compatible Organic Acids Group B – Compatible Pyrophoric & Water Reactive Materials Group C – Compatible Inorganic Bases Group D – Compatible Organic Acids Group E – Compatible Oxidizers including Peroxides Group F– Compatible Inorganic Acids not including Oxidizers or Combustible Group G – Not Intrinsically Reactive or Flammable or Combustible Group J* – Poison Compressed Gases Group K* – Compatible Explosive or other highly Unstable Material Group L – Non-Reactive Flammable and Combustible, including solvents Group X* – Incompatible with ALL other storage groups The following is a list of chemicals and their compatibility storage codes. This is not a complete list of chemicals, but is provided to give examples of each storage group: Storage Group A 94‐75‐7 2,4‐D (2,4‐Dichlorophenoxyacetic acid) 94‐82‐6 2,4‐DB 609-99-4 3,5-Dinitrosalicylic acid 64‐19‐7 Acetic acid (Flammable liquid @ 102°F avoid alcohols, Amines, ox agents see SDS) 631-61-8 Acetic acid, Ammonium salt (Ammonium acetate) 108-24-7 Acetic anhydride (Flammable liquid @102°F avoid alcohols see SDS) 79‐10‐7 Acrylic acid Peroxide Former 65‐85‐0 Benzoic acid 98‐07‐7 Benzotrichloride 98‐88‐4 Benzoyl chloride 107-92-6 Butyric Acid 115‐28‐6 Chlorendic acid 79‐11‐8 Chloroacetic acid 627‐11‐2 Chloroethyl chloroformate 77‐92‐9 Citric acid 5949-29-1 Citric acid monohydrate 57-00-1 Creatine 20624-25-3 -

Insect-Proofing During Building Construction
INSECT-PROOFING DURING BUILDING CONSTRUCTION W. EBELING * R. E. WAGNER - D. A. REIERSON TRUCTURAL AND HOUSEHOLD PEST mental evidence of efficiency of soil treat- foundation walls or piers. Specific in- S CONTROL in the United States re- ment have been the investigations of the structions for treatment for slab-on- quires annual expenditures of hundreds Southern Forest Experiment Station of ground, raised-foundation, and basement of millions of dollars and the services of the Forest Service, U. S. Department of construction may be obtained in publica- 27,000 licensed pest control operators. Agriculture. These investigations showed tions of the federal or state agricultural Preventive measures against structural that currently recommended dosages of experiment stations or among the tech- and household pests should be taken dur- certain chlorinated hydrocarbons formed nical releases of the National Pest Con- ing the construction of a building, be- a compIetely effective barrier against sub- trol Association. cause such measures are most effective terranean termites for a period of 18 and economical at that time. Preventive years in heavily infested forest soil in Prevention measures fall into two general categories, Mississippi, and may continue to be effec- Some househoId pests, including cock- depending on whether they are directed tive for many more years. The pest con- roaches, silverfish, odorous house ants, against (1) subterranean termites, which trol industry has successfully used soil plaster beetles (Lathrididae) , psocids, invade a building from colonies in the treatment for many years. and rat mites, spend all or a major part soil and which first attack in the sub- Currently recommended insecticides, of their time in hidden areas of a house structure; or (2) a number of other in- and concentrations include 0.5 per cent such as the attic, wall voids, soffit voids, sect species that spend either all or a aldrin, dieldrin, or heptachlor, or 1 per or in voids under cabinets or built-in considerable part of their life in the attic, cent chlordane.