Managing Cleft Palates
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Te2, Part Iii
TERMINOLOGIA EMBRYOLOGICA Second Edition International Embryological Terminology FIPAT The Federative International Programme for Anatomical Terminology A programme of the International Federation of Associations of Anatomists (IFAA) TE2, PART III Contents Caput V: Organogenesis Chapter 5: Organogenesis (continued) Systema respiratorium Respiratory system Systema urinarium Urinary system Systemata genitalia Genital systems Coeloma Coelom Glandulae endocrinae Endocrine glands Systema cardiovasculare Cardiovascular system Systema lymphoideum Lymphoid system Bibliographic Reference Citation: FIPAT. Terminologia Embryologica. 2nd ed. FIPAT.library.dal.ca. Federative International Programme for Anatomical Terminology, February 2017 Published pending approval by the General Assembly at the next Congress of IFAA (2019) Creative Commons License: The publication of Terminologia Embryologica is under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license The individual terms in this terminology are within the public domain. Statements about terms being part of this international standard terminology should use the above bibliographic reference to cite this terminology. The unaltered PDF files of this terminology may be freely copied and distributed by users. IFAA member societies are authorized to publish translations of this terminology. Authors of other works that might be considered derivative should write to the Chair of FIPAT for permission to publish a derivative work. Caput V: ORGANOGENESIS Chapter 5: ORGANOGENESIS -

Syndromes of the First and Second Branchial Arches, Part 1: Embryology and Characteristic REVIEW ARTICLE Defects
Syndromes of the First and Second Branchial Arches, Part 1: Embryology and Characteristic REVIEW ARTICLE Defects J.M. Johnson SUMMARY: A variety of congenital syndromes affecting the face occur due to defects involving the G. Moonis first and second BAs. Radiographic evaluation of craniofacial deformities is necessary to define aberrant anatomy, plan surgical procedures, and evaluate the effects of craniofacial growth and G.E. Green surgical reconstructions. High-resolution CT has proved vital in determining the nature and extent of R. Carmody these syndromes. The radiologic evaluation of syndromes of the first and second BAs should begin H.N. Burbank first by studying a series of isolated defects: CL with or without CP, micrognathia, and EAC atresia, which compose the major features of these syndromes and allow more specific diagnosis. After discussion of these defects and the associated embryology, we proceed to discuss the VCFS, PRS, ACS, TCS, Stickler syndrome, and HFM. ABBREVIATIONS: ACS ϭ auriculocondylar syndrome; BA ϭ branchial arch; CL ϭ cleft lip; CL/P ϭ cleft lip/palate; CP ϭ cleft palate; EAC ϭ external auditory canal; HFM ϭ hemifacial microsomia; MDCT ϭ multidetector CT; PRS ϭ Pierre Robin sequence; TCS ϭ Treacher Collins syndrome; VCFS ϭ velocardiofacial syndrome adiographic evaluation of craniofacial deformities is nec- major features of the syndromes of the first and second BAs. Ressary to define aberrant anatomy, plan surgical proce- Part 2 of this review discusses the syndromes and their radio- dures, and evaluate the effects of craniofacial growth and sur- graphic features: PRS, HFM, ACS, TCS, Stickler syndrome, gical reconstructions.1 The recent rapid proliferation of and VCFS. -

Trávicí Systém
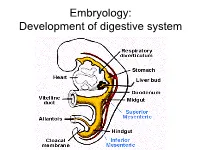
Embryology: Development of digestive system Embryo folding – incorporation of endoderm to form primitive gut. Outside of embryo – yolk sac and allantois. Vitelline duct Stomodeum (primitive mouth) the oral cavity + the salivary glands Proctodeum primitive anal pit Primitive gut whole digestive tube + accessory glands pharynx forgut midgut hindgut • The epithelium and glandular cells of associated glands of the gastrointestinal tract develop from endoderm • The connective tissue, muscle tissue and mesothelium are derived from splanchnic mesoderm • The enteric nervous system develops from neural crest primitive gut foregut midgut hindgut pharyngeal above ductus cloacal membrane omphalomesentericus membrane and yolk sack Derivatives of forgut – pharynx, esophagus (+ respiratory diverticul), stomach, cranial part of duodenum midgut – caudal part of duodenum (+ liver, gall bladder, pancreas), small intestine and part of large intestine (to the flexura coli sin.) hindgut – large intestine (from flexura coli sin.), rectum, upper part of anal canal Oral cavity • primitive mouth pit – stomodeum • lined with ectoderm • surrounded by: - processus frontalis (single) - proc. maxillares (paired) - proc. mandibulares (paired) • pharyngeal membrane (it ruptures during the 4th week, primitive gut communicates with amnionic cavity Pharyngeal (branchial) apparatus Pharyngeal arches • appear in weeks 4 - 5 • on the ventral side of the pharyngeal gut. • each arch has cartilage, cranial nerve, aortic arch artery and muscle • pharyngeal clefts and pouches -
Cleft Palate Are Common Defects That Result in Abnormal Facial Appearance and Defective Speech
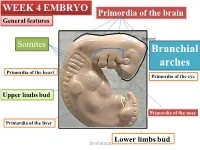
WEEK 4 EMBRYO Primordia of the brain General features shatarat Somites Branchial arches Primordia of the heart Primordia of the eye Upper limbs bud Primordia of the nose Primordia of the liver Lower limbs bud Dr.shatarat The most important feature in the development of the head and neck is the Formation of THE PHARYNGEAL OR BRANCHIALARCHES shatarat Dr.shatarat Is it branchial or is it pharyngeal arch? development of pharyngeal arches resembles formation of gills in fish However, in the human embryo real gills (branchia) are never formed. Therefore, the term pharyngeal arches has been adopted for the human embryo Dr.shatarat shatarat THE PHARYNGEALARCHES appear in the fourth and fifth weeks of development Dr.shatarat Why the appear? Migration of cells from epiblast Dr.shatarat 1-PARAXIALMESODERM 2-LATERALPLATE MESODERM 3-NEURALCREST Dr.shatarat Migration of the cells from the occipital Myotomes into the future mouth to form the tongue Occipital somites This is an explanation to how the arches appear…. as a result of migration of the cells from the medial mesoderm (somites) into tongue the regions of the future head and neck. As we mentioned there are other reasons Dr.shatarat In a cross section of the embryo in the area of the head and neck The following can be noticed THE PHARYNGEALARCHES shatarat THE PHARYNGEALARCHES are separated by deep clefts known as PHARYNGEAL CLEFTS with development of the arches and clefts, a number of outpocketings, The pharyngeal pouches appear Dr.shatarat 1-PHARYNGEAL ARCHs Dr.shatarat 6 However, The fifth and sixth arches are rudimentary and are not visible on the surface of the embryo T h e y a re n u m b e r e d i n c r a n i o ca ud a During the fifth week, the second pharyngeal l s e arch enlarges and overgrows the third and qu fourth arches, forming the ectodermal e n c depression called cervical sinus e Dr.shatarat Each pharyngeal arch consists of: 1-surface ECTODERM 2-a core of MESENCHYMALtissue 3- epithelium of ENDODERMAL origin Each pharyngeal arch contains: 1- An artery that arises from the primordial heart A. -
Ectodermal Pharyngeal Clefts
Embryology: Development of digestive system Flecture of embryo – incorporation of endoderm to form primitive gut. Outside of embryo – yolk sac and allantois. Vitelline duct Stomodeum (primitive mouth) the oral cavity + the salivary glands Proctodeum primitive anal pit Primitive gut whole digestive tube + accessory glands orofacial membrane Tracheo- esophageal septum Proctodeum cloacal membrane Primitive gut pharynx forgut midgut hindgut Derivatives of foregut – pharynx, (+ respiratory diverticle),esophagus, stomach, cranial part of duodenum, (+ liver, gall bladder pancreas) midgut – caudal part of duodenum, small intestine and part of large intestine (1/3 of colon transv.) hindgut – the rest of large intestine, rectum, upper part of the anal canal Tissues in GIT • The epithelium of gut and glandular cells of associated glands of the gastrointestinal tract develop from endoderm • The connective tissue, muscle tissue and mesothelium derive from splanchnic mesoderm • The enteric nervous system develops from neural crest primitive gut foregut midgut hindgut from above ductus to cloacal pharyngeal omphalomesentericus membrane membrane and yolk sack Pharyngeal membrane liver, bile duct, pancreas Rectum Origin: forgut, midgut. hindgut Oral cavity • primitive mouth pit – stomodeum • lined with ectoderm • surrounded by: - processus frontalis (single) - proc. maxillares (paired) - proc. mandibulares (paired) • orophacial membrane (it ruptures during the 4th week, primitive gut communicates with amnionic cavity) Pharyngeal (branchial) apparatus -

Facial and Palatal Development
11. FACIAL AND PALATAL DEVELOPMENT Letty Moss-Salentijn DDS, PhD Dr. Edwin S.Robinson Professor of Dentistry (in Anatomy and Cell Biology) E-mail: [email protected] READING ASSIGNMENT: Larsen 3rd edition: p.352; pp.365-371; 398-404. SUMMARY: The external human face develops between the 4th and 6th weeks of embryonic development. Facial swellings arise on the frontonasal process (2 medial nasal and 2 lateral nasal processes) and the first pharyngeal arch (2 mandibular and 2 maxillary processes). By a process of merging and some localized fusion these processes come together to form the continuous surfaces of the external face. The primary palate is formed in this period by fusion/merging of the medial nasal and maxillary processes. Subsequently, between 6th and 12th embryonic/fetal weeks, the secondary palate is formed as the result of fusion between palatal processes growing from the oral surfaces of the maxillary processes. Each merging and fusion site is also the site of a potential facial or palatal cleft. LEARNING OBJECTIVES You should be able to: a. Demonstrate on a frontal image of a human face those parts of the face that are formed by contributions from the frontonasal process and those that are formed by contributions from the first pharyngeal arch. b. Describe the following as to site, composition, time of appearance, and fate: oropharyngeal membrane, oronasal membrane. c. List the derivatives of the four pairs of facial processes and the pair of palatal processes. d. Describe the development of the nose and primary palate. e. Explain the differences between the processes of merging and fusion. -

High-Yield Embryology 4
LWBK356-FM_pi-xii.qxd 7/14/09 2:03 AM Page i Aptara Inc High-Yield TM Embryology FOURTH EDITION LWBK356-FM_pi-xii.qxd 7/14/09 2:03 AM Page ii Aptara Inc LWBK356-FM_pi-xii.qxd 7/14/09 2:03 AM Page iii Aptara Inc High-Yield TM Embryology FOURTH EDITION Ronald W. Dudek, PhD Professor Brody School of Medicine East Carolina University Department of Anatomy and Cell Biology Greenville, North Carolina LWBK356-FM_pi-xii.qxd 7/14/09 2:03 AM Page iv Aptara Inc Acquisitions Editor: Crystal Taylor Product Manager: Sirkka E. Howes Marketing Manager: Jennifer Kuklinski Vendor Manager: Bridgett Dougherty Manufacturing Manager: Margie Orzech Design Coordinator: Terry Mallon Compositor: Aptara, Inc. Copyright © 2010, 2007, 2001, 1996 Lippincott Williams & Wilkins, a Wolters Kluwer business. 351 West Camden Street 530 Walnut Street Baltimore, MD 21201 Philadelphia, PA 19106 Printed in China All rights reserved. This book is protected by copyright. No part of this book may be reproduced or transmitted in any form or by any means, including as photocopies or scanned-in or other electronic copies, or utilized by any information storage and retrieval system without written permission from the copyright owner, except for brief quotations embodied in critical articles and reviews. Materials appear- ing in this book prepared by individuals as part of their official duties as U.S. government employees are not covered by the above-mentioned copyright. To request permission, please contact Lippincott Williams & Wilkins at 530 Walnut Street, Philadelphia, PA 19106, via email at [email protected], or via website at lww.com (products and services). -

26 April 2010 TE Prepublication Page 1 Nomina Generalia General Terms
26 April 2010 TE PrePublication Page 1 Nomina generalia General terms E1.0.0.0.0.0.1 Modus reproductionis Reproductive mode E1.0.0.0.0.0.2 Reproductio sexualis Sexual reproduction E1.0.0.0.0.0.3 Viviparitas Viviparity E1.0.0.0.0.0.4 Heterogamia Heterogamy E1.0.0.0.0.0.5 Endogamia Endogamy E1.0.0.0.0.0.6 Sequentia reproductionis Reproductive sequence E1.0.0.0.0.0.7 Ovulatio Ovulation E1.0.0.0.0.0.8 Erectio Erection E1.0.0.0.0.0.9 Coitus Coitus; Sexual intercourse E1.0.0.0.0.0.10 Ejaculatio1 Ejaculation E1.0.0.0.0.0.11 Emissio Emission E1.0.0.0.0.0.12 Ejaculatio vera Ejaculation proper E1.0.0.0.0.0.13 Semen Semen; Ejaculate E1.0.0.0.0.0.14 Inseminatio Insemination E1.0.0.0.0.0.15 Fertilisatio Fertilization E1.0.0.0.0.0.16 Fecundatio Fecundation; Impregnation E1.0.0.0.0.0.17 Superfecundatio Superfecundation E1.0.0.0.0.0.18 Superimpregnatio Superimpregnation E1.0.0.0.0.0.19 Superfetatio Superfetation E1.0.0.0.0.0.20 Ontogenesis Ontogeny E1.0.0.0.0.0.21 Ontogenesis praenatalis Prenatal ontogeny E1.0.0.0.0.0.22 Tempus praenatale; Tempus gestationis Prenatal period; Gestation period E1.0.0.0.0.0.23 Vita praenatalis Prenatal life E1.0.0.0.0.0.24 Vita intrauterina Intra-uterine life E1.0.0.0.0.0.25 Embryogenesis2 Embryogenesis; Embryogeny E1.0.0.0.0.0.26 Fetogenesis3 Fetogenesis E1.0.0.0.0.0.27 Tempus natale Birth period E1.0.0.0.0.0.28 Ontogenesis postnatalis Postnatal ontogeny E1.0.0.0.0.0.29 Vita postnatalis Postnatal life E1.0.1.0.0.0.1 Mensurae embryonicae et fetales4 Embryonic and fetal measurements E1.0.1.0.0.0.2 Aetas a fecundatione5 Fertilization -

OKELLO-THESIS.Pdf
Six2 EXHIBITS A TEMPORAL-SPATIAL EXPRESSION PROFILE IN THE DEVELOPING MOUSE PALATE AND IMPACTS CELL PROLIFERATION DURING MURINE PALATOGENESIS A Thesis Submitted to the College of Graduate Studies and Research in Partial Fulfilment of the Requirements for the Degree of Master of Science in the College of Pharmacy and Nutrition University of Saskatchewan Saskatoon By Dennis Okori Okello © Copyright Dennis Okori Okello, July 2015. All rights reserved. PERMISSION TO USE In presenting this thesis in partial fulfilment of the requirements for a Master of Science degree from the University of Saskatchewan, I agree that the Libraries of this University may make it freely available for inspection. I also agree that permission for copying this thesis in any manner, in whole or in part, for scholarly purposes may be granted by Dr. Adil J. Nazarali, the Professor who supervised my thesis work, or in his absence, by the Dean of the College of Pharmacy and Nutrition in which my thesis work was done. It is understood that any copying or publication, or use of this thesis or parts thereof for financial gain shall not be allowed without my written permission. It is also understood that due recognition shall be given to me and to the University of Saskatchewan in any scholarly use which may be made of any material in my thesis. Requests for permission to copy or to make use of material in this thesis in whole or in part should be addressed to: Dean of the College of Pharmacy and Nutrition University of Saskatchewan 104 Clinic Place Saskatoon, Saskatchewan S7N 2Z4, CANADA. -

Development of Face and Palate
Development of Face and Palate Dr. Heba Kalbouneh Associate Professor of Anatomy and Histology Pericardiumbulge Forebrain bulge Dr. Heba Kalbouneh Mandibular process Fronto-nasal process Maxillary process Stomodeum Second arch Pericardial bulge Dr. Heba Kalbouneh Dr. Fronto-nasal process Maxillary process Stomodeum Mandibular process Pericardial bulge Fronto-nasal process Nasal placode Nasal placode Stomodeum On both sides of the frontonasal prominence, local thickenings of the surface ectoderm will be formed, the nasal placodes Medial nasal prominence Nasal pit Lateral nasal prominence Developing eye Nasolacrimal groove Cleft Nasal pit Medial nasal prominence Lateral nasal prominence Nasolacrimal groove Medial nasal prominence Lateral nasal prominence Maxillary process Cleft Frontonasal process Nasal pit Mandibular process Medial nasal prominences fuse and form the middle part of the nose Lateral nasal prominence forms the Naso-lacrimal groove ala of the nose The maxillary process is separated from the side of fronto-nasal process by naso- The two medial lacrimal groove, inside which a cord of ectodermal cells is nasal folds fuse to formed then becomes form the canalized to form naso- Intermaxillary lacrimal duct. Its upper end segment forms lacrimal sac. Naso-lacrimal groove Intermaxillary segment: from fused medial nasal prominences. It forms Intermaxillary segment philtrum, part of upper jaw that carries upper 4 incisors and primary palate Intermaxillary segment: Primary palate Palatine shelves of the maxillary processes: Secondary -

Chapter 12 Head and Neck
LWBK507-c12_p145-155.qxd 11/01/2010 02:02 PM Page 145 Aptara chapter 12 Head and Neck I. PHARYNGEAL APPARATUS (FIGURE 12.1; TABLE 12.1) The pharyngeal apparatus consists of the pharyngeal arches, pharyngeal pouches, pharyngeal grooves, and pharyngeal membranes, all of which contribute greatly to the formation of the head and neck. The pharyngeal apparatus is first observed in week 4 of development and gives the embryo its distinctive appearance. There are five pharyngeal arches (1, 2, 3, 4, and 6), four pha- ryngeal pouches (1, 2, 3, and 4), four pharyngeal grooves (1, 2, 3, and 4), and four pharyngeal membranes (1, 2, 3, and 4). Pharyngeal arch 5 and pharyngeal pouch 5 completely regress in the human. Aortic arch 5 also completely regresses (see Chapter 5). The Hox complex and retinoic acid appear to be important factors in early head and neck formation. A lack or excess of retinoic acid causes striking facial anomalies. A. Pharyngeal arches (1, 2, 3, 4, 6) contain somitomeric mesoderm and neural crest cells. In gen- eral, the mesoderm differentiates into muscles and arteries (i.e., aortic arches 1–6), whereas neural crest cells differentiate into bone and connective tissue. In addition, each pharyngeal arch has a cranial nerve associated with it. B. Pharyngeal pouches (1, 2, 3, 4) are evaginations of endoderm that lines the foregut. C. Pharyngeal grooves (1, 2, 3, 4) are invaginations of ectoderm located between each pharyn- geal arch. D. Pharyngeal membranes (1, 2, 3, 4) are structures consisting of ectoderm, intervening meso- derm and neural crest, and endoderm located between each pharyngeal arch. -

Msx Homeobox Gene Family and Craniofacial Development
Cell research (2003); 13(6):429-442 http://www.cell-research.com REVIEW Msx homeobox gene family and craniofacial development SYLVIA ALAPPAT1, ZUN YI ZHANG1, YI PING CHEN1,2* 1Division of Developmental Biology, Department of Cell and Molecular Biology, Tulane University, New orleans, LA 70118, USA. E-mail: [email protected] 2College of Bioengineering, Fujian Normal University, Fuzhou, Fujian Province, China ABSTRACT Vertebrate Msx genes are unlinked, homeobox-containing genes that bear homology to the Drosophila muscle segment homeobox gene. These genes are expressed at multiple sites of tissue-tissue interactions during verte- brate embryonic development. Inductive interactions mediated by the Msx genes are essential for normal craniofacial, limb and ectodermal organ morphogenesis, and are also essential to survival in mice, as manifested by the pheno- typic abnormalities shown in knockout mice and in humans. This review summarizes studies on the expression, regulation, and functional analysis of Msx genes that bear relevance to craniofacial development in humans and mice. Key words: Msx genes, craniofacial, tooth, cleft palate, suture, development, transcription factor, signaling molecule. INTRODUCTION Msx genes have been isolated from a variety of orga- Vertebrate craniofacial organs form from multiple nisms, including ascidians[5, 6], sea urchin[7], zebrafish embryonic tissues including the cranial neural crest [5, 8], frogs[9], birds[10-12], and humans[13]. The mammalian Msx gene family consists of 3 physically derived cells, prechordal mesoderm, and the embryonic unlinked members, named Msx1, Msx2, and Msx3[14, craniofacial ectoderm. Normal craniofacial morphology 15]. Msx3 is only expressed in the dorsal neural tube, in develops as a consequence of complex interactions a pattern resembling that of the prototypical Drosophila between these embryonic tissues, and requires precise msh gene[16, 17].