An Experimental Investigation of Water Vapor Condensation from Biofuel Flue Gas in a Model of Condenser, (1) Base Case: Local Heat Transfer Without Water Injection
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Soaring Weather
Chapter 16 SOARING WEATHER While horse racing may be the "Sport of Kings," of the craft depends on the weather and the skill soaring may be considered the "King of Sports." of the pilot. Forward thrust comes from gliding Soaring bears the relationship to flying that sailing downward relative to the air the same as thrust bears to power boating. Soaring has made notable is developed in a power-off glide by a conven contributions to meteorology. For example, soar tional aircraft. Therefore, to gain or maintain ing pilots have probed thunderstorms and moun altitude, the soaring pilot must rely on upward tain waves with findings that have made flying motion of the air. safer for all pilots. However, soaring is primarily To a sailplane pilot, "lift" means the rate of recreational. climb he can achieve in an up-current, while "sink" A sailplane must have auxiliary power to be denotes his rate of descent in a downdraft or in come airborne such as a winch, a ground tow, or neutral air. "Zero sink" means that upward cur a tow by a powered aircraft. Once the sailcraft is rents are just strong enough to enable him to hold airborne and the tow cable released, performance altitude but not to climb. Sailplanes are highly 171 r efficient machines; a sink rate of a mere 2 feet per second. There is no point in trying to soar until second provides an airspeed of about 40 knots, and weather conditions favor vertical speeds greater a sink rate of 6 feet per second gives an airspeed than the minimum sink rate of the aircraft. -

Guide to Understanding Condensation
Guide to Understanding Condensation The complete Andersen® Owner-To-Owner™ limited warranty is available at: www.andersenwindows.com. “Andersen” is a registered trademark of Andersen Corporation. All other marks where denoted are marks of Andersen Corporation. © 2007 Andersen Corporation. All rights reserved. 7/07 INTRODUCTION 2 The moisture that suddenly appears in cold weather on the interior We have created this brochure to answer questions you may have or exterior of window and patio door glass can block the view, drip about condensation, indoor humidity and exterior condensation. on the floor or freeze on the glass. It can be an annoying problem. We’ll start with the basics and offer solutions and alternatives While it may seem natural to blame the windows or doors, interior along the way. condensation is really an indication of excess humidity in the home. Exterior condensation, on the other hand, is a form of dew — the Should you run into problems or situations not covered in the glass simply provides a surface on which the moisture can condense. following pages, please contact your Andersen retailer. The important thing to realize is that if excessive humidity is Visit the Andersen website: www.andersenwindows.com causing window condensation, it may also be causing problems elsewhere in your home. Here are some other signs of excess The Andersen customer service toll-free number: 1-888-888-7020. humidity: • A “damp feeling” in the home. • Staining or discoloration of interior surfaces. • Mold or mildew on surfaces or a “musty smell.” • Warped wooden surfaces. • Cracking, peeling or blistering interior or exterior paint. -

Chapter 3 Equations of State
Chapter 3 Equations of State The simplest way to derive the Helmholtz function of a fluid is to directly integrate the equation of state with respect to volume (Sadus, 1992a, 1994). An equation of state can be applied to either vapour-liquid or supercritical phenomena without any conceptual difficulties. Therefore, in addition to liquid-liquid and vapour -liquid properties, it is also possible to determine transitions between these phenomena from the same inputs. All of the physical properties of the fluid except ideal gas are also simultaneously calculated. Many equations of state have been proposed in the literature with either an empirical, semi- empirical or theoretical basis. Comprehensive reviews can be found in the works of Martin (1979), Gubbins (1983), Anderko (1990), Sandler (1994), Economou and Donohue (1996), Wei and Sadus (2000) and Sengers et al. (2000). The van der Waals equation of state (1873) was the first equation to predict vapour-liquid coexistence. Later, the Redlich-Kwong equation of state (Redlich and Kwong, 1949) improved the accuracy of the van der Waals equation by proposing a temperature dependence for the attractive term. Soave (1972) and Peng and Robinson (1976) proposed additional modifications of the Redlich-Kwong equation to more accurately predict the vapour pressure, liquid density, and equilibria ratios. Guggenheim (1965) and Carnahan and Starling (1969) modified the repulsive term of van der Waals equation of state and obtained more accurate expressions for hard sphere systems. Christoforakos and Franck (1986) modified both the attractive and repulsive terms of van der Waals equation of state. Boublik (1981) extended the Carnahan-Starling hard sphere term to obtain an accurate equation for hard convex geometries. -

Effect of Wind on Near-Shore Breaking Waves
EFFECT OF WIND ON NEAR-SHORE BREAKING WAVES by Faydra Schaffer A Thesis Submitted to the Faculty of The College of Engineering and Computer Science in Partial Fulfillment of the Requirements for the Degree of Master of Science Florida Atlantic University Boca Raton, Florida December 2010 Copyright by Faydra Schaffer 2010 ii ACKNOWLEDGEMENTS The author wishes to thank her mother and family for their love and encouragement to go to college and be able to have the opportunity to work on this project. The author is grateful to her advisor for sponsoring her work on this project and helping her to earn a master’s degree. iv ABSTRACT Author: Faydra Schaffer Title: Effect of wind on near-shore breaking waves Institution: Florida Atlantic University Thesis Advisor: Dr. Manhar Dhanak Degree: Master of Science Year: 2010 The aim of this project is to identify the effect of wind on near-shore breaking waves. A breaking wave was created using a simulated beach slope configuration. Testing was done on two different beach slope configurations. The effect of offshore winds of varying speeds was considered. Waves of various frequencies and heights were considered. A parametric study was carried out. The experiments took place in the Hydrodynamics lab at FAU Boca Raton campus. The experimental data validates the knowledge we currently know about breaking waves. Offshore winds effect is known to increase the breaking height of a plunging wave, while also decreasing the breaking water depth, causing the wave to break further inland. Offshore winds cause spilling waves to react more like plunging waves, therefore increasing the height of the spilling wave while consequently decreasing the breaking water depth. -

NWS Unified Surface Analysis Manual
Unified Surface Analysis Manual Weather Prediction Center Ocean Prediction Center National Hurricane Center Honolulu Forecast Office November 21, 2013 Table of Contents Chapter 1: Surface Analysis – Its History at the Analysis Centers…………….3 Chapter 2: Datasets available for creation of the Unified Analysis………...…..5 Chapter 3: The Unified Surface Analysis and related features.……….……….19 Chapter 4: Creation/Merging of the Unified Surface Analysis………….……..24 Chapter 5: Bibliography………………………………………………….…….30 Appendix A: Unified Graphics Legend showing Ocean Center symbols.….…33 2 Chapter 1: Surface Analysis – Its History at the Analysis Centers 1. INTRODUCTION Since 1942, surface analyses produced by several different offices within the U.S. Weather Bureau (USWB) and the National Oceanic and Atmospheric Administration’s (NOAA’s) National Weather Service (NWS) were generally based on the Norwegian Cyclone Model (Bjerknes 1919) over land, and in recent decades, the Shapiro-Keyser Model over the mid-latitudes of the ocean. The graphic below shows a typical evolution according to both models of cyclone development. Conceptual models of cyclone evolution showing lower-tropospheric (e.g., 850-hPa) geopotential height and fronts (top), and lower-tropospheric potential temperature (bottom). (a) Norwegian cyclone model: (I) incipient frontal cyclone, (II) and (III) narrowing warm sector, (IV) occlusion; (b) Shapiro–Keyser cyclone model: (I) incipient frontal cyclone, (II) frontal fracture, (III) frontal T-bone and bent-back front, (IV) frontal T-bone and warm seclusion. Panel (b) is adapted from Shapiro and Keyser (1990) , their FIG. 10.27 ) to enhance the zonal elongation of the cyclone and fronts and to reflect the continued existence of the frontal T-bone in stage IV. -

Chapter 3 Bose-Einstein Condensation of an Ideal
Chapter 3 Bose-Einstein Condensation of An Ideal Gas An ideal gas consisting of non-interacting Bose particles is a ¯ctitious system since every realistic Bose gas shows some level of particle-particle interaction. Nevertheless, such a mathematical model provides the simplest example for the realization of Bose-Einstein condensation. This simple model, ¯rst studied by A. Einstein [1], correctly describes important basic properties of actual non-ideal (interacting) Bose gas. In particular, such basic concepts as BEC critical temperature Tc (or critical particle density nc), condensate fraction N0=N and the dimensionality issue will be obtained. 3.1 The ideal Bose gas in the canonical and grand canonical ensemble Suppose an ideal gas of non-interacting particles with ¯xed particle number N is trapped in a box with a volume V and at equilibrium temperature T . We assume a particle system somehow establishes an equilibrium temperature in spite of the absence of interaction. Such a system can be characterized by the thermodynamic partition function of canonical ensemble X Z = e¡¯ER ; (3.1) R where R stands for a macroscopic state of the gas and is uniquely speci¯ed by the occupa- tion number ni of each single particle state i: fn0; n1; ¢ ¢ ¢ ¢ ¢ ¢g. ¯ = 1=kBT is a temperature parameter. Then, the total energy of a macroscopic state R is given by only the kinetic energy: X ER = "ini; (3.2) i where "i is the eigen-energy of the single particle state i and the occupation number ni satis¯es the normalization condition X N = ni: (3.3) i 1 The probability -

Liquid-Vapor Equilibrium in a Binary System
Liquid-Vapor Equilibria in Binary Systems1 Purpose The purpose of this experiment is to study a binary liquid-vapor equilibrium of chloroform and acetone. Measurements of liquid and vapor compositions will be made by refractometry. The data will be treated according to equilibrium thermodynamic considerations, which are developed in the theory section. Theory Consider a liquid-gas equilibrium involving more than one species. By definition, an ideal solution is one in which the vapor pressure of a particular component is proportional to the mole fraction of that component in the liquid phase over the entire range of mole fractions. Note that no distinction is made between solute and solvent. The proportionality constant is the vapor pressure of the pure material. Empirically it has been found that in very dilute solutions the vapor pressure of solvent (major component) is proportional to the mole fraction X of the solvent. The proportionality constant is the vapor pressure, po, of the pure solvent. This rule is called Raoult's law: o (1) psolvent = p solvent Xsolvent for Xsolvent = 1 For a truly ideal solution, this law should apply over the entire range of compositions. However, as Xsolvent decreases, a point will generally be reached where the vapor pressure no longer follows the ideal relationship. Similarly, if we consider the solute in an ideal solution, then Eq.(1) should be valid. Experimentally, it is generally found that for dilute real solutions the following relationship is obeyed: psolute=K Xsolute for Xsolute<< 1 (2) where K is a constant but not equal to the vapor pressure of pure solute. -
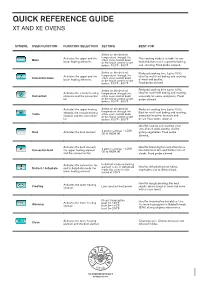
QUICK REFERENCE GUIDE XT and XE Ovens
QUICK REFERENCE GUIDE XT AND XE OVENS SYMBOL OVEN FUNCTION FUNCTION SELECTION SETTING BEST FOR Select on the desired Activates the upper and the temperature through the This cooking mode is suitable for any Bake either oven control knob lower heating elements or the touch control smart kind of dishes and it is great for baking botton. 100°F - 500°F and roasting. Food probe allowed. Select on the desired Reduced cooking time (up to 10%). Activates the upper and the temperature through the Ideal for multi-level baking and roasting Convection bake either oven control knob lower heating elements or the touch control smart of meat and poultry. botton. 100°F - 500°F Food probe allowed. Select on the desired Reduced cooking time (up to 10%). Activates the circular heating temperature through the Ideal for multi-level baking and roasting, Convection elements and the convection either oven control knob especially for cakes and pastry. Food fan or the touch control smart probe allowed. botton. 100°F - 500°F Activates the upper heating Select on the desired Reduced cooking time (up to 10%). temperature through the Best for multi-level baking and roasting, Turbo element, the circular heating either oven control knob element and the convection or the touch control smart especially for pizza, focaccia and fan botton. 100°F - 500°F bread. Food probe allowed. Ideal for searing and roasting small cuts of beef, pork, poultry, and for 4 power settings – LOW Broil Activates the broil element (1) to HIGH (4) grilling vegetables. Food probe allowed. Activates the broil element, Ideal for browning fish and other items 4 power settings – LOW too delicate to turn and thicker cuts of Convection broil the upper heating element (1) to HIGH (4) and the convection fan steaks. -

It's Just a Phase!
BASIS Lesson Plan Lesson Name: It’s Just a Phase! Grade Level Connection(s) NGSS Standards: Grade 2, Physical Science FOSS CA Edition: Grade 3 Physical Science: Matter and Energy Module *Note to teachers: Detailed standards connections can be found at the end of this lesson plan. Teaser/Overview Properties of matter are illustrated through a series of demonstrations and hands-on explorations. Students will learn to identify solids, liquids, and gases. Water will be used to demonstrate the three phases. Students will learn about sublimation through a fun experiment with dry ice (solid CO2). Next, they will compare the propensity of several liquids to evaporate. Finally, they will learn about freezing and melting while making ice cream. Lesson Objectives ● Students will be able to identify the three states of matter (solid, liquid, gas) based on the relative properties of those states. ● Students will understand how to describe the transition from one phase to another (melting, freezing, evaporation, condensation, sublimation) ● Students will learn that matter can change phase when heat/energy is added or removed Vocabulary Words ● Solid: A phase of matter that is characterized by a resistance to change in shape and volume. ● Liquid: A phase of matter that is characterized by a resistance to change in volume; a liquid takes the shape of its container ● Gas: A phase of matter that can change shape and volume ● Phase change: Transformation from one phase of matter to another ● Melting: Transformation from a solid to a liquid ● Freezing: Transformation -

A STUDY of ELEVATED CONVECTION and ITS IMPACTS on SURFACE WEATHER CONDITIONS a Dissertat
A STUDY OF ELEVATED CONVECTION AND ITS IMPACTS ON SURFACE WEATHER CONDITIONS _______________________________________ A Dissertation presented to the Faculty of the Graduate School at the University of Missouri-Columbia _______________________________________________________ In Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy _____________________________________________________ by Joshua Kastman Dr. Patrick Market, Dissertation Supervisor December 2017 © copyright by Joshua S. Kastman 2017 All Rights Reserved The undersigned, appointed by the dean of the Graduate School, have examined the dissertation entitled A Study of Elevated Convection and its Impacts on Surface Weather Conditions presented by Joshua Kastman, a candidate for the degree of doctor of philosophy, Soil, Environmental, and Atmospheric Sciences and hereby certify that, in their opinion, it is worthy of acceptance. ____________________________________________ Professor Patrick Market ____________________________________________ Associate Professor Neil Fox ____________________________________________ Professor Anthony Lupo ____________________________________________ Associate Professor Sonja Wilhelm Stannis ACKNOWLEDGMENTS I would like to begin by thanking Dr. Patrick Market for all of his guidance and encouragement throughout my time at the University of Missouri. His advice has been invaluable during my graduate studies. His mentorship has meant so much to me and I look forward his advice and friendship in the years to come. I would also like to thank Anthony Lupo, Neil Fox and Sonja Wilhelm-Stannis for serving as committee members and for their advice and guidance. I would like to thank the National Science Foundation for funding the project. I would l also like to thank my wife Anna for her, support, patience and understanding while I pursued this degree. Her love and partnerships means everything to me. -

Capillary Condensation of Colloid–Polymer Mixtures Confined
INSTITUTE OF PHYSICS PUBLISHING JOURNAL OF PHYSICS: CONDENSED MATTER J. Phys.: Condens. Matter 15 (2003) S3411–S3420 PII: S0953-8984(03)66420-9 Capillary condensation of colloid–polymer mixtures confined between parallel plates Matthias Schmidt1,Andrea Fortini and Marjolein Dijkstra Soft Condensed Matter, Debye Institute, Utrecht University, Princetonplein 5, 3584 CC Utrecht, The Netherlands Received 21 July 2003 Published 20 November 2003 Onlineatstacks.iop.org/JPhysCM/15/S3411 Abstract We investigate the fluid–fluid demixing phase transition of the Asakura–Oosawa model colloid–polymer mixture confined between two smooth parallel hard walls using density functional theory and computer simulations. Comparing fluid density profiles for statepoints away from colloidal gas–liquid coexistence yields good agreement of the theoretical results with simulation data. Theoretical and simulation results predict consistently a shift of the demixing binodal and the critical point towards higher polymer reservoir packing fraction and towards higher colloid fugacities upon decreasing the plate separation distance. This implies capillary condensation of the colloid liquid phase, which should be experimentally observable inside slitlike micropores in contact with abulkcolloidal gas. 1. Introduction Capillary condensation denotes the phenomenon that spatial confinement can stabilize a liquid phase coexisting with its vapour in bulk [1, 2]. In order for this to happen the attractive interaction between the confining walls and the fluid particles needs to be sufficiently strong. Although the main body of work on this subject has been done in the context of confined simple liquids, one might expect that capillary condensation is particularly well suited to be studied with colloidal dispersions. In these complex fluids length scales are on the micron rather than on the ångstrom¨ scale which is typical for atomicsubstances. -

Alside Condensation Guide Download
Condensation Your Guide To Common Household Condensation C Understanding Condensation Common household condensation, or “sweating” on windows is caused by excess humidity or water vapor in a home. When this water vapor in the air comes in contact with a cold surface such as a mirror or window glass, it turns to water droplets and is called condensation. Occasional condensation, appearing as fog on the windows or glass surfaces, is normal and is no cause for concern. On the other hand, excessive window Conquering the Myth . Windows Ccondensation, frost, peeling paint, or Do Not Cause Condensation. moisture spots on ceilings and walls can be signs of potentially damaging humidity You may be wondering why your new levels in your home. We tend to notice energy-efficient replacement windows show condensation on windows and mirrors first more condensation than your old drafty ones. because moisture doesn’t penetrate these Your old windows allowed air to flow between surfaces. Yet they are not the problem, simply the inside of your home and the outdoors. the indicators that you need to reduce the Your new windows create a tight seal between indoor humidity of your home. your home and the outside. Excess moisture is unable to escape, and condensation becomes visible. Windows do not cause condensation, but they are often one of the first signs of excessive humidity in the air. Understanding Condensation Where Does Indoor Outside Recommended Humidity Come From? Temperature Relative Humidity All air contains a certain amount of moisture, +20°F 30% - 35% even indoors. And there are many common +10°F 25% - 30% things that generate indoor humidity such as your heating system, humidifiers, cooking and 0°F 20% - 25% showers.