Chapter 3 Bose-Einstein Condensation of an Ideal
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lecture 3: Fermi-Liquid Theory 1 General Considerations Concerning Condensed Matter
Phys 769 Selected Topics in Condensed Matter Physics Summer 2010 Lecture 3: Fermi-liquid theory Lecturer: Anthony J. Leggett TA: Bill Coish 1 General considerations concerning condensed matter (NB: Ultracold atomic gasses need separate discussion) Assume for simplicity a single atomic species. Then we have a collection of N (typically 1023) nuclei (denoted α,β,...) and (usually) ZN electrons (denoted i,j,...) interacting ∼ via a Hamiltonian Hˆ . To a first approximation, Hˆ is the nonrelativistic limit of the full Dirac Hamiltonian, namely1 ~2 ~2 1 e2 1 Hˆ = 2 2 + NR −2m ∇i − 2M ∇α 2 4πǫ r r α 0 i j Xi X Xij | − | 1 (Ze)2 1 1 Ze2 1 + . (1) 2 4πǫ0 Rα Rβ − 2 4πǫ0 ri Rα Xαβ | − | Xiα | − | For an isolated atom, the relevant energy scale is the Rydberg (R) – Z2R. In addition, there are some relativistic effects which may need to be considered. Most important is the spin-orbit interaction: µ Hˆ = B σ (v V (r )) (2) SO − c2 i · i × ∇ i Xi (µB is the Bohr magneton, vi is the velocity, and V (ri) is the electrostatic potential at 2 3 2 ri as obtained from HˆNR). In an isolated atom this term is o(α R) for H and o(Z α R) for a heavy atom (inner-shell electrons) (produces fine structure). The (electron-electron) magnetic dipole interaction is of the same order as HˆSO. The (electron-nucleus) hyperfine interaction is down relative to Hˆ by a factor µ /µ 10−3, and the nuclear dipole-dipole SO n B ∼ interaction by a factor (µ /µ )2 10−6. -

Guide to Understanding Condensation
Guide to Understanding Condensation The complete Andersen® Owner-To-Owner™ limited warranty is available at: www.andersenwindows.com. “Andersen” is a registered trademark of Andersen Corporation. All other marks where denoted are marks of Andersen Corporation. © 2007 Andersen Corporation. All rights reserved. 7/07 INTRODUCTION 2 The moisture that suddenly appears in cold weather on the interior We have created this brochure to answer questions you may have or exterior of window and patio door glass can block the view, drip about condensation, indoor humidity and exterior condensation. on the floor or freeze on the glass. It can be an annoying problem. We’ll start with the basics and offer solutions and alternatives While it may seem natural to blame the windows or doors, interior along the way. condensation is really an indication of excess humidity in the home. Exterior condensation, on the other hand, is a form of dew — the Should you run into problems or situations not covered in the glass simply provides a surface on which the moisture can condense. following pages, please contact your Andersen retailer. The important thing to realize is that if excessive humidity is Visit the Andersen website: www.andersenwindows.com causing window condensation, it may also be causing problems elsewhere in your home. Here are some other signs of excess The Andersen customer service toll-free number: 1-888-888-7020. humidity: • A “damp feeling” in the home. • Staining or discoloration of interior surfaces. • Mold or mildew on surfaces or a “musty smell.” • Warped wooden surfaces. • Cracking, peeling or blistering interior or exterior paint. -

Phys 446: Solid State Physics / Optical Properties Lattice Vibrations
Solid State Physics Lecture 5 Last week: Phys 446: (Ch. 3) • Phonons Solid State Physics / Optical Properties • Today: Einstein and Debye models for thermal capacity Lattice vibrations: Thermal conductivity Thermal, acoustic, and optical properties HW2 discussion Fall 2007 Lecture 5 Andrei Sirenko, NJIT 1 2 Material to be included in the test •Factors affecting the diffraction amplitude: Oct. 12th 2007 Atomic scattering factor (form factor): f = n(r)ei∆k⋅rl d 3r reflects distribution of electronic cloud. a ∫ r • Crystalline structures. 0 sin()∆k ⋅r In case of spherical distribution f = 4πr 2n(r) dr 7 crystal systems and 14 Bravais lattices a ∫ n 0 ∆k ⋅r • Crystallographic directions dhkl = 2 2 2 1 2 ⎛ h k l ⎞ 2πi(hu j +kv j +lw j ) and Miller indices ⎜ + + ⎟ •Structure factor F = f e ⎜ a2 b2 c2 ⎟ ∑ aj ⎝ ⎠ j • Definition of reciprocal lattice vectors: •Elastic stiffness and compliance. Strain and stress: definitions and relation between them in a linear regime (Hooke's law): σ ij = ∑Cijklε kl ε ij = ∑ Sijklσ kl • What is Brillouin zone kl kl 2 2 C •Elastic wave equation: ∂ u C ∂ u eff • Bragg formula: 2d·sinθ = mλ ; ∆k = G = eff x sound velocity v = ∂t 2 ρ ∂x2 ρ 3 4 • Lattice vibrations: acoustic and optical branches Summary of the Last Lecture In three-dimensional lattice with s atoms per unit cell there are Elastic properties – crystal is considered as continuous anisotropic 3s phonon branches: 3 acoustic, 3s - 3 optical medium • Phonon - the quantum of lattice vibration. Elastic stiffness and compliance tensors relate the strain and the Energy ħω; momentum ħq stress in a linear region (small displacements, harmonic potential) • Concept of the phonon density of states Hooke's law: σ ij = ∑Cijklε kl ε ij = ∑ Sijklσ kl • Einstein and Debye models for lattice heat capacity. -

Particle Characterisation In
LIFE SCIENCE I TECHNICAL BULLETIN ISSUE N°11 /JULY 2008 PARTICLE CHARACTERISATION IN EXCIPIENTS, DRUG PRODUCTS AND DRUG SUBSTANCES AUTHOR: HILDEGARD BRÜMMER, PhD, CUSTOMER SERVICE MANAGER, SGS LIFE SCIENCE SERVICES, GERMANY Particle characterization has significance in many industries. For the pharmaceutical industry, particle size impacts products in two ways: as an influence on drug performance and as an indicator of contamination. This article will briefly examine how particle size impacts both, and review the arsenal of methods for measuring and tracking particle size. Furthermore, examples of compromised product quality observed within our laboratories illustrate why characterization is so important. INDICATOR OF CONTAMINATION Controlling the limits of contaminating • Adverse indirect reactions: particles Particle characterisation of drug particles is critical for injectable are identified by the immune system as substances, drug products and excipients (parenteral) solutions. Particle foreign material and immune reaction is an important factor in R&D, production contamination of solutions can potentially might impose secondary effects. and quality control of pharmaceuticals. have the following results: It is becoming increasingly important for In order to protect a patient and to compliance with requirements of FDA and • Adverse direct reactions: e.g. particles guarantee a high quality product, several European Health Authorities. are distributed via the blood in the body chapters in the compendia (USP, EP, JP) and cause toxicity to specific tissues or describe techniques for characterisation organs, or particles of a given size can of limits. Some of the most relevant cause a physiological effect blocking blood chapters are listed in Table 1. flow e.g. in the lungs. -
![Arxiv:2102.13616V2 [Cond-Mat.Quant-Gas] 30 Jul 2021 That Preserve Stability of the Underlying Problem1](https://docslib.b-cdn.net/cover/6442/arxiv-2102-13616v2-cond-mat-quant-gas-30-jul-2021-that-preserve-stability-of-the-underlying-problem1-456442.webp)
Arxiv:2102.13616V2 [Cond-Mat.Quant-Gas] 30 Jul 2021 That Preserve Stability of the Underlying Problem1
Self-stabilized Bose polarons Richard Schmidt1, 2 and Tilman Enss3 1Max-Planck-Institute of Quantum Optics, Hans-Kopfermann-Straße 1, 85748 Garching, Germany 2Munich Center for Quantum Science and Technology, Schellingstraße 4, 80799 Munich, Germany 3Institut f¨urTheoretische Physik, Universit¨atHeidelberg, 69120 Heidelberg, Germany (Dated: August 2, 2021) The mobile impurity in a Bose-Einstein condensate (BEC) is a paradigmatic many-body problem. For weak interaction between the impurity and the BEC, the impurity deforms the BEC only slightly and it is well described within the Fr¨ohlich model and the Bogoliubov approximation. For strong local attraction this standard approach, however, fails to balance the local attraction with the weak repulsion between the BEC particles and predicts an instability where an infinite number of bosons is attracted toward the impurity. Here we present a solution of the Bose polaron problem beyond the Bogoliubov approximation which includes the local repulsion between bosons and thereby stabilizes the Bose polaron even near and beyond the scattering resonance. We show that the Bose polaron energy remains bounded from below across the resonance and the size of the polaron dressing cloud stays finite. Our results demonstrate how the dressing cloud replaces the attractive impurity potential with an effective many-body potential that excludes binding. We find that at resonance, including the effects of boson repulsion, the polaron energy depends universally on the effective range. Moreover, while the impurity contact is strongly peaked at positive scattering length, it remains always finite. Our solution highlights how Bose polarons are self-stabilized by repulsion, providing a mechanism to understand quench dynamics and nonequilibrium time evolution at strong coupling. -

It's Just a Phase!
BASIS Lesson Plan Lesson Name: It’s Just a Phase! Grade Level Connection(s) NGSS Standards: Grade 2, Physical Science FOSS CA Edition: Grade 3 Physical Science: Matter and Energy Module *Note to teachers: Detailed standards connections can be found at the end of this lesson plan. Teaser/Overview Properties of matter are illustrated through a series of demonstrations and hands-on explorations. Students will learn to identify solids, liquids, and gases. Water will be used to demonstrate the three phases. Students will learn about sublimation through a fun experiment with dry ice (solid CO2). Next, they will compare the propensity of several liquids to evaporate. Finally, they will learn about freezing and melting while making ice cream. Lesson Objectives ● Students will be able to identify the three states of matter (solid, liquid, gas) based on the relative properties of those states. ● Students will understand how to describe the transition from one phase to another (melting, freezing, evaporation, condensation, sublimation) ● Students will learn that matter can change phase when heat/energy is added or removed Vocabulary Words ● Solid: A phase of matter that is characterized by a resistance to change in shape and volume. ● Liquid: A phase of matter that is characterized by a resistance to change in volume; a liquid takes the shape of its container ● Gas: A phase of matter that can change shape and volume ● Phase change: Transformation from one phase of matter to another ● Melting: Transformation from a solid to a liquid ● Freezing: Transformation -

Scientific and Related Works of Chen Ning Yang
Scientific and Related Works of Chen Ning Yang [42a] C. N. Yang. Group Theory and the Vibration of Polyatomic Molecules. B.Sc. thesis, National Southwest Associated University (1942). [44a] C. N. Yang. On the Uniqueness of Young's Differentials. Bull. Amer. Math. Soc. 50, 373 (1944). [44b] C. N. Yang. Variation of Interaction Energy with Change of Lattice Constants and Change of Degree of Order. Chinese J. of Phys. 5, 138 (1944). [44c] C. N. Yang. Investigations in the Statistical Theory of Superlattices. M.Sc. thesis, National Tsing Hua University (1944). [45a] C. N. Yang. A Generalization of the Quasi-Chemical Method in the Statistical Theory of Superlattices. J. Chem. Phys. 13, 66 (1945). [45b] C. N. Yang. The Critical Temperature and Discontinuity of Specific Heat of a Superlattice. Chinese J. Phys. 6, 59 (1945). [46a] James Alexander, Geoffrey Chew, Walter Salove, Chen Yang. Translation of the 1933 Pauli article in Handbuch der Physik, volume 14, Part II; Chapter 2, Section B. [47a] C. N. Yang. On Quantized Space-Time. Phys. Rev. 72, 874 (1947). [47b] C. N. Yang and Y. Y. Li. General Theory of the Quasi-Chemical Method in the Statistical Theory of Superlattices. Chinese J. Phys. 7, 59 (1947). [48a] C. N. Yang. On the Angular Distribution in Nuclear Reactions and Coincidence Measurements. Phys. Rev. 74, 764 (1948). 2 [48b] S. K. Allison, H. V. Argo, W. R. Arnold, L. del Rosario, H. A. Wilcox and C. N. Yang. Measurement of Short Range Nuclear Recoils from Disintegrations of the Light Elements. Phys. Rev. 74, 1233 (1948). [48c] C. -

2. Classical Gases
2. Classical Gases Our goal in this section is to use the techniques of statistical mechanics to describe the dynamics of the simplest system: a gas. This means a bunch of particles, flying around in a box. Although much of the last section was formulated in the language of quantum mechanics, here we will revert back to classical mechanics. Nonetheless, a recurrent theme will be that the quantum world is never far behind: we’ll see several puzzles, both theoretical and experimental, which can only truly be resolved by turning on ~. 2.1 The Classical Partition Function For most of this section we will work in the canonical ensemble. We start by reformu- lating the idea of a partition function in classical mechanics. We’ll consider a simple system – a single particle of mass m moving in three dimensions in a potential V (~q ). The classical Hamiltonian of the system3 is the sum of kinetic and potential energy, p~ 2 H = + V (~q ) 2m We earlier defined the partition function (1.21) to be the sum over all quantum states of the system. Here we want to do something similar. In classical mechanics, the state of a system is determined by a point in phase space.Wemustspecifyboththeposition and momentum of each of the particles — only then do we have enough information to figure out what the system will do for all times in the future. This motivates the definition of the partition function for a single classical particle as the integration over phase space, 1 3 3 βH(p,q) Z = d qd pe− (2.1) 1 h3 Z The only slightly odd thing is the factor of 1/h3 that sits out front. -

Particle Number Concentrations and Size Distribution in a Polluted Megacity
https://doi.org/10.5194/acp-2020-6 Preprint. Discussion started: 17 February 2020 c Author(s) 2020. CC BY 4.0 License. Particle number concentrations and size distribution in a polluted megacity: The Delhi Aerosol Supersite study Shahzad Gani1, Sahil Bhandari2, Kanan Patel2, Sarah Seraj1, Prashant Soni3, Zainab Arub3, Gazala Habib3, Lea Hildebrandt Ruiz2, and Joshua S. Apte1 1Department of Civil, Architectural and Environmental Engineering, The University of Texas at Austin, Texas, USA 2McKetta Department of Chemical Engineering, The University of Texas at Austin, Texas, USA 3Department of Civil Engineering, Indian Institute of Technology Delhi, New Delhi, India Correspondence: Joshua S. Apte ([email protected]), Lea Hildebrandt Ruiz ([email protected]) Abstract. The Indian national capital, Delhi, routinely experiences some of the world's highest urban particulate matter concentrations. While fine particulate matter (PM2.5) mass concentrations in Delhi are at least an order of magnitude higher than in many western cities, the particle number (PN) concentrations are not similarly elevated. Here we report on 1.25 years of highly 5 time resolved particle size distributions (PSD) data in the size range of 12–560 nm. We observed that the large number of accumulation mode particles—that constitute most of the PM2.5 mass—also contributed substantially to the PN concentrations. The ultrafine particles (UFP, Dp <100 nm) fraction of PN was higher during the traffic rush hours and for daytimes of warmer seasons—consistent with traffic and nucleation events being major sources of urban UFP. UFP concentrations were found to be relatively lower during periods with some of the highest mass concentrations. -

High Pressure Band Structure, Density of States, Structural Phase Transition and Metallization in Cds
Chemical and Materials Engineering 5(1): 8-13, 2017 http://www.hrpub.org DOI: 10.13189/cme.2017.050102 High Pressure Band Structure, Density of States, Structural Phase Transition and Metallization in CdS J. Jesse Pius1, A. Lekshmi2, C. Nirmala Louis2,* 1Rohini College of Engineering, Nagercoil, Kanyakumari District, India 2Research Center in Physics, Holy Cross College, India Copyright©2017 by authors, all rights reserved. Authors agree that this article remains permanently open access under the terms of the Creative Commons Attribution License 4.0 International License Abstract The electronic band structure, density of high pressure studies due to the development of different states, metallization and structural phase transition of cubic designs of diamond anvil cell (DAC). With a modern DAC, zinc blende type cadmium sulphide (CdS) is investigated it is possible to reach pressures of 2 Mbar (200 GPa) using the full potential linear muffin-tin orbital (FP-LMTO) routinely and pressures of 5 Mbar (500 GPa) or higher is method. The ground state properties and band gap values achievable [3]. At such pressures, materials are reduced to are compared with the experimental results. The fractions of their original volumes. With this reduction in equilibrium lattice constant, bulk modulus and its pressure inter atomic distances; significant changes in bonding and derivative and the phase transition pressure at which the structure as well as other properties take place. The increase compounds undergo structural phase transition from ZnS to of pressure means the significant decrease in volume, which NaCl are predicted from the total energy calculations. The results in the change of electronic states and crystal structure. -

The Two-Dimensional Bose Gas in Box Potentials Laura Corman
The two-dimensional Bose Gas in box potentials Laura Corman To cite this version: Laura Corman. The two-dimensional Bose Gas in box potentials. Physics [physics]. Université Paris sciences et lettres, 2016. English. NNT : 2016PSLEE014. tel-01449982 HAL Id: tel-01449982 https://tel.archives-ouvertes.fr/tel-01449982 Submitted on 30 Jan 2017 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. THÈSE DE DOCTORAT de l’Université de recherche Paris Sciences Lettres – PSL Research University préparée à l’École normale supérieure The Two-Dimensional Bose École doctorale n°564 Gas in Box Potentials Spécialité: Physique Soutenue le 02.06.2016 Composition du Jury : par Laura Corman M Tilman Esslinger ETH Zürich Rapporteur Mme Hélène Perrin Université Paris XIII Rapporteur M Zoran Hadzibabic Cambridge University Membre du Jury M Gilles Montambaux Université Paris XI Membre du Jury M Jean Dalibard Collège de France Directeur de thèse M Jérôme Beugnon Université Paris VI Membre invité ABSTRACT Degenerate atomic gases are a versatile tool to study many-body physics. They offer the possibility to explore low-dimension physics, which strongly differs from the three dimensional (3D) case due to the enhanced role of fluctuations. -
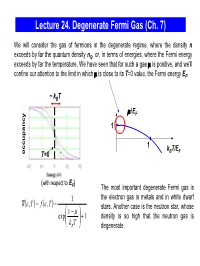
Lecture 24. Degenerate Fermi Gas (Ch
Lecture 24. Degenerate Fermi Gas (Ch. 7) We will consider the gas of fermions in the degenerate regime, where the density n exceeds by far the quantum density nQ, or, in terms of energies, where the Fermi energy exceeds by far the temperature. We have seen that for such a gas μ is positive, and we’ll confine our attention to the limit in which μ is close to its T=0 value, the Fermi energy EF. ~ kBT μ/EF 1 1 kBT/EF occupancy T=0 (with respect to E ) F The most important degenerate Fermi gas is 1 the electron gas in metals and in white dwarf nε()(),, T= f ε T = stars. Another case is the neutron star, whose ε⎛ − μ⎞ exp⎜ ⎟ +1 density is so high that the neutron gas is ⎝kB T⎠ degenerate. Degenerate Fermi Gas in Metals empty states ε We consider the mobile electrons in the conduction EF conduction band which can participate in the charge transport. The band energy is measured from the bottom of the conduction 0 band. When the metal atoms are brought together, valence their outer electrons break away and can move freely band through the solid. In good metals with the concentration ~ 1 electron/ion, the density of electrons in the electron states electron states conduction band n ~ 1 electron per (0.2 nm)3 ~ 1029 in an isolated in metal electrons/m3 . atom The electrons are prevented from escaping from the metal by the net Coulomb attraction to the positive ions; the energy required for an electron to escape (the work function) is typically a few eV.