FRANJE 36 Najaar 2015
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

PACIFIC INSECTS MONOGRAPH Ll
PACIFIC INSECTS MONOGRAPH ll Lepidoptera of American Samoa with particular reference to biology and ecology By John Adams Comstock Published by Entomology Department, Bernice P. Bishop Museum Honolulu, Hawaii, U. S. A. 1966 PACIFIC INSECTS MONOGRAPHS Published by Entomology Department, Bernice P. Bishop Museum, Honolulu, Hawaii, 96819, U. S. A. Editorial Committee: J. L. Gressitt, Editor (Honolulu), S. Asahina (Tokyo), R. G. Fennah (London), R. A. Harrison (Christchurch), T. C. Maa (Honolulu & Taipei), C. W. Sabrosky (Washington, D. C), R. L. Usinger (Berkeley), J. van der Vecht (Leiden), K. Yasumatsu (Fukuoka), E. C. Zimmerman (New Hampshire). Assistant Editors: P. D. Ashlock (Honolulu), Carol Higa (Honolulu), Naoko Kunimori (Fukuoka), Setsuko Nakata (Honolulu), Toshi Takata (Fukuoka). Business Manager: C. M. Yoshimoto (Honolulu). Business Assistant: Doris Anbe (Honolulu). Business Agent in Japan: K. Yasumatsu (Fukuoka). Entomological staff, Bishop Museum, 1966: Doris Anbe, Hatsuko Arakaki, P. D. Ashlock, S. Azuma, Madaline Boyes, Candida Cardenas, Ann Cutting, M. L. Goff, J. L. Gressitt (Chairman), J. Harrell, Carol Higa, Y. Hirashima, Shirley Hokama, E. Holzapfel, Dorothy Hoxie, Helen Hurd, June Ibara, Naoko Kuni mori, T. C. Maa, Grace Nakahashi, Setsuko Nakata (Adm. Asst.), Tulene Nonomura, Carol Okuma, Ka tharine Pigue, Linda Reineccius, T. Saigusa, I. Sakakibara, Judy Sakamoto, G. A. Samuelson, Sybil Seto, W. A. Steffan, Amy Suehiro, Grace Thompson, Clara Uchida, J. R. Vockeroth, Nixon Wilson, Mabel Ya- tsuoka, C. M. Yoshimoto, E. C. Zimmermann. Field associates: M. J. Fitzsimons, E. E. Gless, G. E. Lip- pert, V. Peckham, D. S. Rabor, J. Sedlacek, M. Sedlacek, P. Shanahan, R. Straatman, J. Strong, H. M. Tor- revillas, A. -
R Graphics Output
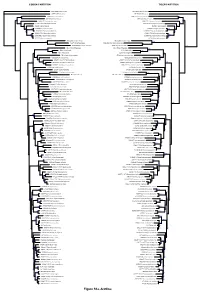
CODON PARTITION TIGER PARTITION SIR14GEO Setina irrorella SIR14GEO Setina irrorella AMSP13GE1 Amata sp. AMSP13GE1 Amata sp. 94 MM05671 Coscinia cribraria MM05671 Coscinia cribraria 94 DNA1252 Tyria jacobaeae DNA1252 Tyria jacobaeae 73 78 NSGJZ017 Aglaomorpha histrio NSGJZ017 Aglaomorpha histrio 92 95 CPUD13SPA Cymbalophora pudica CPUD13SPA Cymbalophora pudica 95 92 JMZI001 Haploa reversa JMZI001 Haploa reversa 75 100 KN00905 Euplagia splendidior KN00905 Euplagia splendidior 100 88 70 NSGJZ003 Euplagia quadripunctata NSGJZ003 Euplagia quadripunctata 52 CDOM14FIN Callimorpha dominula CDOM14FIN Callimorpha dominula 100 100 100 CDOM14GE1 Callimorpha dominula CDOM14GE1 Callimorpha dominula 100 RZ136 Callimorpha dominula RZ136 Callimorpha dominula NSGJZ021 Secusio doriae NSGJZ021 Secusio doriae 100 100 89 RZ387 Nyctemera baulus NSGJZ008 Nyctemera adversata 90 98 NSGJZ008 Nyctemera adversata RZ387 Nyctemera baulus 100 KC33 Virbia immaculata KC33 Virbia immaculata 100 100 99 JZ000 Virbia fragilis KC11 Virbia opella 100 69 KC11 Virbia opella JZ000 Virbia fragilis 61 RZ30 Creatonotos transiens RZ30 Creatonotos transiens 99 100 NSGJZ009 Chionarctia nivea NSGJZ009 Chionarctia nivea 81 72 CNLEP113666 Pyrrharctia isabella CNLEP113666 Pyrrharctia isabella 100 100 100 100 81 NSGJZ011 Phragmatobia amuriensis KN00881 Amurrhyparia leopardinula 85 KN00881 Amurrhyparia leopardinula NSGJZ011 Phragmatobia amuriensis SCDNA169 Arachnis picta SCDNA169 Arachnis picta 99 CR055 Hypercompe laeta CR055 Hypercompe laeta 61 56 97 100 T1 Estigmene tenuistrigata T1 Estigmene -

Do Jumping Spiders (Araneae: Salticidae) Draw Their Own Portraits?
Peckhamia 179.1 Self portraits by jumping spiders 1 PECKHAMIA 179.1, 6 February 2019, 1―14 ISSN 2161―8526 (print) urn:lsid:zoobank.org:pub:6116EC94-2146-49D9-8FDE-F11833CFA03D (registered 31 JAN 2019) ISSN 1944―8120 (online) Do jumping spiders (Araneae: Salticidae) draw their own portraits? David E. Hill,1 Abhijith A. P. C.2 and João P. Burini 3 1 213 Wild Horse Creek Drive, Simpsonville SC 29680, USA, email [email protected] 2 Indraprastha Organic Farm, Kalalwadi Village, Udboor Post, Mysuru-570008, Karnataka, India, email abhiapc@ gmail.com 3 São Paulo, Brazil, email [email protected] Abstract. Many different insects appear to mimic the appearance of the salticid spiders as viewed from the front. Examples of this mimicry are reviewed with respect to the hypothesis that these are examples of predator mimicry, whereby salticid spiders are less likely to attack prey that present images of other salticid spiders. Key words. Anastrepha, Batesian mimicry, Blattodea, Brenthia, Brixia, Ceratitis, Choreutis, Chrysops, Cixiidae, Derbidae, Fulgoroidea, Glyphipterigidae, Glyphipterix, Goniurella, Graphopsocus, Leptoceridae, metalmark moths, Mimarachne, mimicry, Nectopsyche, Olethreutes, Phidippus, Platensina, Plexippus, predator mimicry, Procecidochares, Psocoptera, Rhagoletis, Rhotana, Rhotanini, Saltissus, Stenopsocidae, Tabanidae, Tephritidae, Tortricidae, Trichoptera, Tritoxa, Trupanea, Zonosemata Many insects display an image that suggests the appearance of a salticid spider as viewed from the front. In some cases this display also includes movement suggestive of the aggressive or agonistic displays of these spiders (e.g., Lim & Li 2004; Hill 2018). Here we review a series of examples that may represent predator mimicry, or the mimicry of predators, in this case salticid spiders, by their prey. -

Dancing Behavior of Cosmopterix Victor STRINGER, a Species New to the Fauna of China
Beitr. Ent. Keltern ISSN 0005 - 805X Beitr. Ent. 58 (2008) 1 205 58 (2008) 1 S. 205 - 210 15.07.2008 Dancing behavior of Cosmopterix victor STRINGER, a species new to the fauna of China (Lepidoptera: Cosmopterigidae) With 3 figures STEVEN R. DAVIS and SERGEY YU. SINEV Summary A bamboo-feeding species Cosmopterix victor STRINGER, 1930 is mentioned from mainland Asia for the first time, with a description and illustration of its very striking dancing behavior. This behavior is regarded as a special adaptation providing the aggregation of adults on the host plants for mating and subsequent oviposition. Keywords Microlepidoptera, Zhejiang Province, Cosmopterigidae, Cosmopterix victor, dancing behavior. Zusammenfassung Zum ersten Mal wird ein Fund der Bambus fressenden Art Cosmopterix victor STRINGER, 1930 vom asiati- schen Festland gemeldet. Weiterhin wird das sehr auffällige Tanzverhalten dieser Art beschrieben und abgebildet. Dabei handelt es sich um eine spezielle Anpassung, um für eine Ansammlung der adulten Schmetterlinge auf der Wirtspflanze zur Paarung und anschließenden Eiablage zu sorgen. Introduction The genus Cosmopterix is an easily recognized group, composed of approximately 185 species worldwide, and distinct from other Cosmopterigidae by its characteristic wing pattern (SINEV 2002; KOSTER and SINEV 2003; KUROKO and LIU 2005). Although many species are nearly identical in wing pattern, their genital morphology bears striking differences (HODGES 1962; SINEV 1985, 1988, 1997). The East Asian fauna of Cosmopterix has been reviewed mainly by SINEV and KUROKO, and the Chinese fauna is in great need of further systematic study. In the summer of 2006 the first author spent approximately 30 minutes on Tian Mu mountain (Zhejiang Province, China) observing several specimens of Cosmopterix dancing on the leaves of unknown species of bamboo (Poaceae: Bambuseae). -

Lepidoptera: Gelechiidae) from Costa Rica
28 March 2006 PROC. ENTOMOL. SOc. WASH. 108(2), 2006, pp. 253-260 TAXONOMIC AND BEHAVIORAL STUDIES OF A NEW DANCING BELTHECA BUSCK (LEPIDOPTERA: GELECHIIDAE) FROM COSTA RICA AKlTO Y. KAWAHARA AND DAVID ADAMSKl (AYK) Maryland Center for Systematic Entomology, University of Maryland, 4112 Plant Sciences Building, College Park, MD 20742-4454, U.S.A. (e-mail: kawahara@ umd.edu); (DA) Department of Entomology, National Museum of Natural History, Smith sonian Institution, Washington, D.C. 20013-7012, U.S.A. (e-mail: [email protected]. usda.gov) Abstract.-Beltheca oni, new species, is described from the Atlantic lowlands of Costa Rica. The adult female is similar to that of B. picolella Busck, but the former lacks a signum. The male can be distinguished from that of Beltheca phosphoropa (Meyrick) by its longer vinculum and larger aedeagus. Adults of both sexes "dance" on leaves of different plants by anchoring one of their forelegs to a substrate and rotating around this pivot point. The anchored leg shifts slightly when the moth dances in large circles, moves to another region of the leaf, or changes the direction of rotation. There is no preference for rotating clockwise or counterclockwise. Dancing occurs all over the adaxial leaf sur face, but it can be localized. An adult was observed dancing around a water droplet and drinking from it. We hypothesize that dancing is a courtship behavior or a predator avoid ance tactic. A photograph of the adult and illustrations of the head, wing venation, and genitalia of both sexes are included along with a diagram of a dancing path. -

Beginner S Guide to Moths of the Midwest Micromoths
0LGZHVW5HJLRQ86$ %HJLQQHU V*XLGHWR0RWKVRIWKH0LGZHVW0LFURPRWKV $QJHOOD0RRUHKRXVH ,OOLQRLV1DWXUH3UHVHUYH&RPPLVVLRQ Photos: Angella Moorehouse ([email protected]). Produced by: Angella Moorehouse with the assistance of Alicia Diaz, Field Museum. Identification assistance provided by: multiple sources (inaturalist.org; bugguide.net) )LHOG0XVHXP &&%<1&/LFHQVHGZRUNVDUHIUHHWRXVHVKDUHUHPL[ZLWKDWWULEXWLRQEXWFRPPHUFLDOXVHRIWKHRULJLQDOZRUN LVQRWSHUPLWWHG >ILHOGJXLGHVILHOGPXVHXPRUJ@>@YHUVLRQ $ERXWWKH%(*,11(5¶6027+62)7+(0,':(67*8,'(6 Most photos were taken in west-central and central Illinois; a few are from eastern Iowa and north-central Wisconsin. Nearly all were posted to identification websites: BugGuide.netDQG iNaturalist.org. Identification help was provided by Aaron Hunt, Steve Nanz, John and Jane Balaban, Chris Grinter, Frank Hitchell, Jason Dombroskie, William H. Taft, Jim Wiker,DQGTerry Harrison as well as others contributing to the websites. Attempts were made to obtain expert verifications for all photos to the field identification level, however, there will be errors. Please contact the author with all corrections Additional assistance was provided by longtime Lepidoptera survey partner, Susan Hargrove. The intention of these guides is to provide the means to compare photographs of living specimens of related moths from the Midwest to aid the citizen scientists with identification in the field for Bio Blitz, Moth-ers Day, and other night lighting events. A taxonomic list to all the species featured is provided at the end along with some field identification tips. :(%6,7(63529,',1*,'(17,),&$7,21,1)250$7,21 BugGuide.net iNaturalist.org Mothphotographersgroup.msstate.edu Insectsofiowa.org centralillinoisinsects.org/weblog/resources/ :+,&+027+*8,'(7286( The moths were split into 6 groups for the purposes of creating smaller guides focusing on similar features of 1 or more superfamilies. -

45565782010.Pdf
SHILAP Revista de lepidopterología ISSN: 0300-5267 ISSN: 2340-4078 [email protected] Sociedad Hispano-Luso-Americana de Lepidopterología España Falck, P.; Karsholt, O.; Rota, J. A new species of Anthophila Haworth, 1811 with variable male genitalia from the Canary Islands (Spain) (Lepidoptera: Choreutidae) SHILAP Revista de lepidopterología, vol. 48, no. 192, 2020, October-, pp. 671-681 Sociedad Hispano-Luso-Americana de Lepidopterología España Available in: https://www.redalyc.org/articulo.oa?id=45565782010 How to cite Complete issue Scientific Information System Redalyc More information about this article Network of Scientific Journals from Latin America and the Caribbean, Spain and Journal's webpage in redalyc.org Portugal Project academic non-profit, developed under the open access initiative SHILAP Revta. lepid., 48 (192) diciembre 2020: 671-681 eISSN: 2340-4078 ISSN: 0300-5267 A new species of Anthophila Haworth, 1811 with variable male genitalia from the Canary Islands (Spain) (Lepidoptera: Choreutidae) P. Falck, O. Karsholt & J. Rota Abstract We describe and illustrate Anthophila variabilis Falck, Karsholt & Rota, sp. n. (Choreutidae) from Tenerife (Canary Islands, Spain). The new species is outstanding due to the variability of its male genitalia. It is closely related to A. fabriciana (Linnaeus, 1767), and more distantly related to Anthophila threnodes (Walsingham, 1910), which is endemic to Madeira. Based on the DNA barcode, the new species is molecularly very distinct from its closest relative, A. fabriciana, with a pairwise K2P distance of more than 6.5%. The previous record of A. fabriciana from the Canary Islands is based on misidentification, and the species should be removed from the list of Lepidoptera found in the Canary Islands. -

Journal of the Lepidopterists' Society
J OURNAL OF T HE L EPIDOPTERISTS’ S OCIETY Volume 62 2008 Number 3 Journal of the Lepidopterists’ Society 62(3), 2008, 121-129 IMMATURE STAGES OF METALMARK MOTHS FROM THE GENUS BRENTHIA CLEMENS (CHOREUTIDAE): MORPHOLOGY AND LIFE HISTORY NOTES JADRANKA ROTA Department of Ecology and Evolutionary Biology, University of Connecticut, Storrs, CT 06269, USA; Current address: Department of Entomology, National Museum of Natural History, Smithsonian Institution, Washington, DC 20013-7012; email: [email protected] ABSTRACT. In this paper the immature stages of Brenthia monolychna Meyrick (Choreutidae: Brenthiinae), as well as their ul- trastructure, are described and figured. This is the first description of a New World brenthiine. In addition, notes on life history for four New World species of Brenthia Clemens are provided, including mention of their host plants and parasitoids. Host plant uti- lization of the genus is discussed. A clarification of the nomenclature of the longest seta on the larval abdominal segment 9 is pro- posed. Earlier literature disagrees on whether this is a lateral, subdorsal, or dorsal seta – my examination suggests it is the subdorsal seta 1. The recorded distribution of Brenthia pavonacella Clemens is questioned, and a revised distribution is suggested. Moreover, an escape mechanism, employed by all known Brenthia larvae, is discussed. Finally, a list of morphological and behavioral synapo- morphies for the subfamily Brenthiinae and the genus Brenthia is provided. Additional key words: Microlepidoptera, chaetotaxy, ultrastructure, larval escape behavior, parasitoids, Braconidae Brenthia Clemens is a cosmopolitan genus of mechanism (see below; Williams 1951; Diakonoff 1986; metalmark moths (Choreutidae). With more than 80 Aiello and Solis 2003). -

Nota Lepidopterologica
©Societas Europaea Lepidopterologica; download unter http://www.biodiversitylibrary.org/ und www.zobodat.at Lepi. 2014: 91-103 10.3897/nl.37.7928 Nota 37(1) | DOI Choreutidae of Madeira: review of the known species and description of the male oiAnthophila threnodes (Walsingham, 1910) (Lepidoptera) Jadranka Rota^ Antonio M. F. Aguiar^ Ole Karsholt^ 1 Laboratory of Genetics/Zoological Museum, Department ofBiology, University of Turku, FI-20014 Turku, Finland; jadranka. [email protected] 2 Laboratorio de Qualidade Agricola, Entomologia, Caminho Municipal dos Caboucos, 61, 9135-372 Camacha, Madeira, Portugal; [email protected] 3 Zoological Museum, University of Copenhagen, Universitetsparken 15, DK-2 100 Copenhagen, Denmark; okarsholt@snm. ku. dk http://zoobank. org/9CD3F560-D46D-4E63-A309-E74D061 799E7 Received 13 March 2014; accepted 10 May 2014; published: 15 June 2014 Subject Editor: Erik van Nieukerken Abstract. We review and illustrate the four species of Choreutidae recorded from Madeira - Anthophila threnodes (Walsingham), A. fabriciana (Linnaeus), Choreutis nemorana (Hübner), and Tebenna micalis (Mann) - and describe and illustrate for the first time the male of A. threnodes, as well as the biology of this Madeiran endemic. We provide brief notes on each of the species and give short diagnoses for cor- rectly identifying them. Finally, we discuss previous misidentifications of Madeiran choreutids and the occurrence of choreutids on other oceanic islands. Introduction The Lepidoptera fauna of the Madeira Islands consists of only 331 species (Aguiar & Karsholt 2008). This is mainly due to the isolated position of these islands in the Atlantic Ocean, and only to a lesser extent to insufficient collecting efforts. The Macrolepidoptera fauna, and es- pecially the butterflies (Papilionoidea), are considered to be well known, with only a few and mostly invasive species being added in recent years. -

Fülogeneetiline Uurimus Rööviku Karvasuse Seosest Hoiatusvärvuse, Toitumistüübi Ja Termoregulatsiooniga
TARTU ÜLIKOOL ÖKOLOOGIA JA MAATEADUSTE INSTITUUT ZOOLOOGIA OSAKOND ENTOMOLOOGIA ÕPPETOOL Daniel Valdma Karvasus kui röövikute enesekaitsestrateegia: fülogeneetiline uurimus rööviku karvasuse seosest hoiatusvärvuse, toitumistüübi ja termoregulatsiooniga Magistritöö Juhendaja: Siiri-Lii Sandre TARTU 2016 Infoleht Magistritööle “Karvasus kui röövikute enesekaitsestrateegia: fülogeneetiline uurimus karvasuse seosest hoiatusvärvusega, toitumistüübiga ja termoregulatsiooniga”. Röövikud on toiduallikaks mitmetele erinevatele loomadele. Näiteks putuktoidulised linnud, seemnetoiduliste lindude pojad, erinevad röövtoidulised putukad etc . Röövikutel on mitmeid erinevaid kaitsestrateegiaid röövloomade vastu. Kaitsestrateegiaks võivad olla keemiline kaitse, mehaaniline kaitse, käitumuslik kaitse või hoiatavad signaalid. Tavaliselt on keemiline kaitse esindatud läbi aposemaatilise värvuse. Karvad, harjased ja ogad on efektiivsed kaitsemeetmed lindude ja putukate vastu, aga osad linnud ja parasitoidid on spetsialiseerunud hästi kaitstud karvastele röövikutele. Käesolev fülogeneetiline analüüs näitab, et röövikute karvu enamasti signaliseeritakse aposemaatilise värvusega. Hoiatusvärvid on näitamaks, et karvased röövikud on söögiks kõlbmatud või ohtlik toiduallikas. Hoiatusvärvid karvastel röövikutel ei näita ainult potentsiaalse keemilise kaitse olemasolu vaid annavad märku karvadest kui mehaanilisest kaitsest. Vaatamata sellele, et karvad võivad pakkuda röövikule termoisolatsiooni ning karvased röövikud on pigem mustade toonidega, ei olnud röövikuna -

Metalmark Moths Mimic Their Jumping Spider Predators Jadranka Rota*, David L
Predator Mimicry: Metalmark Moths Mimic Their Jumping Spider Predators Jadranka Rota*, David L. Wagner Department of Ecology and Evolutionary Biology, University of Connecticut, Storrs, Connecticut, United States of America Cases of mimicry provide many of the nature’s most convincing examples of natural selection. Here we report evidence for a case of predator mimicry in which metalmark moths in the genus Brenthia mimic jumping spiders, one of their predators. In controlled trials, Brenthia had higher survival rates than other similarly sized moths in the presence of jumping spiders and jumping spiders responded to Brenthia with territorial displays, indicating that Brenthia were sometimes mistaken for jumping spiders, and not recognized as prey. Our experimental results and a review of wing patterns of other insects indicate that jumping spider mimicry is more widespread than heretofore appreciated, and that jumping spiders are probably an important selective pressure shaping the evolution of diurnal insects that perch on vegetation. Citation: Rota J, Wagner DL (2006) Predator Mimicry: Metalmark Moths Mimic Their Jumping Spider Predators. PLoS ONE 1(1): e45. doi:10.1371/ journal.pone.0000045 INTRODUCTION involving salticid spiders, but in this case salticids are predators The phenomenon of mimicry, a high degree of resemblance due to and not prey. selection, was first proposed in 1862 by Sir Walter Henry Bates Jumping spiders (Araneae: Salticidae) are visual predators of upon his return from eleven years as a professional collector in small arthropods. Among the cues that salticids use for Amazon. Writing about butterfly wing patterns, Bates noted, ‘‘… distinguishing between prey, mates, rivals, and enemies are shape, on these expanded membranes Nature writes, as on a tablet, the symmetry, presence of legs and wings, size, and style of motion story of the modifications of species…’’ Bates proposed that [12]. -

Multicomponent Traits Enhance the Success of Mimicry in Spider-Mimicking Moths
Animal Behaviour 127 (2017) 219e224 Contents lists available at ScienceDirect Animal Behaviour journal homepage: www.elsevier.com/locate/anbehav Sheep in wolf's clothing: multicomponent traits enhance the success of mimicry in spider-mimicking moths * * Mu-Yun Wang a, b, , Vera Vasas c, Lars Chittka c, Shen-Horn Yen a, a Department of Biological Sciences, National Sun Yat-sen University, Kaohsiung, Taiwan b Graduate School of Arts and Sciences, The University of Tokyo, Tokyo, Japan c Department of Biological and Experimental Psychology, School of Biological and Chemical Sciences, Queen Mary, University of London, London, U.K. article info Predator mimicry occurs when prey resemble their predator to gain protection. We explored the relative Article history: importance of the morphological and locomotor signals that spider-mimicking moths use to deceive Received 17 October 2016 their jumping spider predators. Two hypotheses explain why animals use multicomponent signals for Initial acceptance 15 December 2016 communication: the ‘back-up signal’ hypothesis which suggests that multiple traits increase accuracy, Final acceptance 27 February 2017 and the ‘multiple message’ hypothesis which suggests that the different traits serve different purposes or target different signal receivers. We conducted predation tests using the putative spider-mimicking moths Brenthia coronigera (visual and locomotor mimicry) and Choreutis hyligenes (only locomotor MS. number: 16-00911R mimicry) and a control moth species displaying no mimicry. We found that B. coronigera used multi- component signals, i.e. pattern, display posture and jumping behaviour, to deceive its jumping spider Keywords: predators, and thus experienced lower predation rates and more time for escaping. Spiders suffered a Batesian mimicry decreased predation rate when they encountered B.