Chest Imaging Using Signs, Symbols, and Naturalistic Images
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
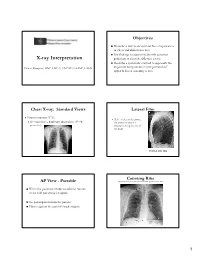
X-Ray Interpretation
Objectives Describe a systematic method for interpretation of chest and abdomen x-rays List findings to accurately identify common X-ray Interpretation pathology in chest & abdomen x-rays Describe a systematic method to approach the Denise Ramponi, DNP, FNP-C, ENP-BC, FAANP, FAEN important components in interpretation of upper & lower extremity x-rays Chest X-ray: Standard Views Lateral Film Postero-anterior (PA): (LAT) view can determine th On inspiration – diaphragm descends to 10 rib the anterior-posterior posteriorly structures along the axis of the body Normal LAT film Counting Ribs AP View - Portable http://www.lumen.luc.edu/lumen/MedEd/medicine/pulmonar/cxr/cxr_f.htm When the patient is unable to tolerate routine views with pts sitting or supine No participation from the patient Film is against the patient's back (supine) 1 Consolidation, Atelectasis, Chest radiograph Interstitial involvement Consolidation - any pathologic process that fills the alveoli with Left and right heart fluid, pus, blood, cells or other borders well defined substances Interstitial - involvement of the Both hemidiaphragms supporting tissue of the lung visible to midline parenchyma resulting in fine or coarse reticular opacities Right - higher Atelectasis - collapse of a part of Heart less than 50% of the lung due to a decrease in the amount of air resulting in volume diameter of the chest loss and increased density. Infiltrate, Consolidation vs. Congestive Heart Failure Atelectasis Fluid leaking into interstitium Kerley B 2 Kerley B lines Prominent interstitial markings Kerley lines Magnified CXR Cardiomyopathy & interstitial pulmonary edema Short 1-2 cm white lines at lung periphery horizontal to pleural surface Distended interlobular septa - secondary to interstitial edema. -

Update on Diagnosis and Treatment of Idiopathic Pulmonary Fibrosis
J Bras Pneumol. 2015;41(5):454-466 http://dx.doi.org/10.1590/S1806-37132015000000152 REVIEW ARTICLE Update on diagnosis and treatment of idiopathic pulmonary fibrosis José Baddini-Martinez1, Bruno Guedes Baldi2, Cláudia Henrique da Costa3, Sérgio Jezler4, Mariana Silva Lima5, Rogério Rufino3,6 1. Divisão de Pneumologia, Departamento de Clínica Médica, Faculdade de Medicina de Ribeirão Preto, Universidade de São Paulo, Ribeirão Preto, Brasil. 2. Divisão de Pneumologia, Instituto do Coração, Hospital das Clínicas, ABSTRACT Faculdade de Medicina, Universidade de São Paulo, São Paulo, Brasil. Idiopathic pulmonary fibrosis is a type of chronic fibrosing interstitial pneumonia, of 3. Disciplina de Pneumologia e Tisiologia, unknown etiology, which is associated with a progressive decrease in pulmonary Faculdade de Ciências Médicas, function and with high mortality rates. Interest in and knowledge of this disorder have Universidade do Estado do Rio de grown substantially in recent years. In this review article, we broadly discuss distinct Janeiro, Rio de Janeiro, Brasil. aspects related to the diagnosis and treatment of idiopathic pulmonary fibrosis. We 4. Ambulatório de Pneumologia, Hospital list the current diagnostic criteria and describe the therapeutic approaches currently Ana Nery, Salvador, Brasil. available, symptomatic treatments, the action of new drugs that are effective in slowing 5. Ambulatório de Doenças Pulmonares Intersticiais, Hospital do Servidor the decline in pulmonary function, and indications for lung transplantation. Público Estadual de São Paulo, São Keywords: Idiopathic pulmonary fibrosis/diagnosis; Idiopathic pulmonary fibrosis/therapy; Paulo, Brasil. Idiopathic pulmonary fibrosis/rehabilitation. 6. Programa de Pós-Graduação em Ciências Médicas, Universidade do Estado do Rio de Janeiro, Rio de Janeiro, Brasil. -

Chest and Abdominal Radiograph 101
Chest and Abdominal Radiograph 101 Ketsia Pierre MD, MSCI July 16, 2010 Objectives • Chest radiograph – Approach to interpreting chest films – Lines/tubes – Pneumothorax/pneumomediastinum/pneumopericar dium – Pleural effusion – Pulmonary edema • Abdominal radiograph – Tubes – Bowel gas pattern • Ileus • Bowel obstruction – Pneumoperitoneum First things first • Turn off stray lights, optimize room lighting • Patient Data – Correct patient – Patient history – Look at old films • Routine Technique: AP/PA, exposure, rotation, supine or erect Approach to Reading a Chest Film • Identify tubes and lines • Airway: trachea midline or deviated, caliber change, bronchial cut off • Cardiac silhouette: Normal/enlarged • Mediastinum • Lungs: volumes, abnormal opacity or lucency • Pulmonary vessels • Hila: masses, lymphadenopathy • Pleura: effusion, thickening, calcification • Bones/soft tissues (four corners) Anatomy of a PA Chest Film TUBES Endotracheal Tubes Ideal location for ETT Is 5 +/‐ 2 cm from carina ‐Normal ETT excursion with flexion and extension of neck 2 cm. ETT at carina Right mainstem Intubation ‐Right mainstem intubation with left basilar atelectasis. ETT too high Other tubes to consider DHT down right mainstem DHT down left mainstem NGT with tip at GE junction CENTRAL LINES Central Venous Line Ideal location for tip of central venous line is within superior vena cava. ‐ Risk of thrombosis decreased in central veins. ‐ Catheter position within atrium increases risk of perforation Acceptable central line positions • Zone A –distal SVC/superior atriocaval junction. • Zone B – proximal SVC • Zone C –left brachiocephalic vein. Right subclavian central venous catheter directed cephalad into IJ Where is this tip? Hemiazygous Or this one? Right vertebral artery Pulmonary Arterial Catheter Ideal location for tip of PA catheter within mediastinal shadow. -

Cardiothoracic Fellowship Program
Cardiothoracic Fellowship Program Table of Contents Program Contact ............................................................................................ 3 Other contact numbers .................................................................................. 4 Introduction ........................................................................................................... 5 Goals and Objectives of Fellowship: ..................................................................... 6 Rotation Schedule: ........................................................................................ 7 Core Curriculum .................................................................................................... 8 Fellow’s Responsibilities ..................................................................................... 22 Resources ........................................................................................................... 23 Facilities ....................................................................................................... 23 Educational Program .......................................................................................... 26 Duty Hours .......................................................................................................... 29 Evaluation ........................................................................................................... 30 Table of Appendices .................................................................................... 31 Appendix A -

Screening Chest X-Ray Interpretations and Radiographic Techniques IOM GUIDELINES FIRST EDITION Iii
FIRST EDITION 2015 Screening Chest X-Ray Interpretations and Radiographic Techniques IOM GUIDELINES Global Radiology Coordination and Teleradiology Centre Migration Health Division International Organization for Migration (Manila Administrative Centre) 24th floor Citibank Tower, Paseo De Roxas 8741, Makati city 1226 Metro Manila, Philippines Email: [email protected] • [email protected] Tel: +632 230 1674 The opinions expressed in the report are those of the authors and do not necessarily reflect the views of the International Organization for Migration (IOM). The designations employed and the presentation of material throughout the report do not imply the expression of any opinion whatsoever on the part of IOM concerning the legal status of any country, territory, city or area, or of its authorities, or concerning its frontiers or boundaries. IOM is committed to the principle that humane and orderly migration benefits migrants and society. As an intergovernmental organization, IOM acts with its partners in the international community to: assist in meeting the operational challenges of migration; advance understanding of migration issues; encourage social and economic development through migration; and uphold the human dignity and well-being of migrants. Author Sifrash Meseret GELAW, MD Radiologist, MPH; Global Radiology Coordinator IOM, Manila Administrative Centre, Manila, Philippines Major Contributor Anthony MACDERMOTT, MD former Global HAP Quality Coordinator, IOM, Regional Office for Asia and the Pacific, Bangkok, Thailand Additional -
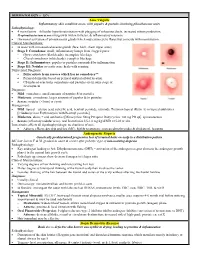
Pediatrics-EOR-Outline.Pdf
DERMATOLOGY – 15% Acne Vulgaris Inflammatory skin condition assoc. with papules & pustules involving pilosebaceous units Pathophysiology: • 4 main factors – follicular hyperkeratinization with plugging of sebaceous ducts, increased sebum production, Propionibacterium acnes overgrowth within follicles, & inflammatory response • Hormonal activation of pilosebaceous glands which may cause cyclic flares that coincide with menstruation Clinical Manifestations: • In areas with increased sebaceous glands (face, back, chest, upper arms) • Stage I: Comedones: small, inflammatory bumps from clogged pores - Open comedones (blackheads): incomplete blockage - Closed comedones (whiteheads): complete blockage • Stage II: Inflammatory: papules or pustules surrounded by inflammation • Stage III: Nodular or cystic acne: heals with scarring Differential Diagnosis: • Differentiate from rosacea which has no comedones** • Perioral dermatitis based on perioral and periorbital location • CS-induced acne lacks comedones and pustules are in same stage of development Diagnosis: • Mild: comedones, small amounts of papules &/or pustules • Moderate: comedones, larger amounts of papules &/or pustules • Severe: nodular (>5mm) or cystic Management: • Mild: topical – azelaic acid, salicylic acid, benzoyl peroxide, retinoids, Tretinoin topical (Retin A) or topical antibiotics [Clindamycin or Erythromycin with Benzoyl peroxide] • Moderate: above + oral antibiotics [Minocycline 50mg PO qd or Doxycycline 100 mg PO qd], spironolactone • Severe (refractory nodular acne): oral -

Mantke, Peitz, Surgical Ultrasound -- Index
419 Index A esophageal 218 Anorchidism 376 gallbladder 165 Aorta 364–366 A-mode imaging 97 gastric 220 abdominal aneurysm (AAA) AAA (abdominal aortic aneurysm) metastasis 142 20–21, 364, 366 20–21, 364, 366 pancreatic 149, 225 dissection 364, 366 Abdominal wall Adenofibroma, breast 263 perforation 366 abscess 300–301 Adenoma pseudoaneurysm 364 diagnostic evaluation 297 adrenal 214 Aortic rupture 20 hematoma 73, 300, 305 colorectal 231, 232 Aplasia, muscular 272 rectus sheath 297–300 duodenal papilla 229, 231 Appendicitis 1–4 hernia 300, 302–304 gallbladder 165 consequences for surgical indications for sonography 297 hepatic 54, 58, 141 treatment 2 seroma 298, 300, 305 multiple 141 sonographic criteria 1 trauma 297–300 parathyroid 213 Archiving 418 Abortion, tubal 30 renal 241 Arteriosclerosis 346, 348 Abscess thyroid 202–203 carotid artery 335, 337, 338 abdominal wall 300–301 Adenomyomatosis 8, 164, 165 plaque 337, 338, 345, 367, 370 causes 301 Adrenal glands 214–216 Arteriovenous (AV) malformation amebic 138 adenoma 214 139, 293, 326–329 breast 264 carcinoma 214 Artery chest wall 173, 178 cyst 214 carotid 334–339 diverticular 120, 123 hematoma 214 aneurysm 338 drainage 85–88, 93 hemorrhage 214 arteriosclerosis 335 hepatic 6, 138, 398 hyperplasia 214 plaque characteristics inflammatory bowel disease limpoma/myelipoma 214 337, 338, 345 116, 119 metastases 214 bifurcation 334, 337 intramural 5 sonographic criteria 214 bulb 339 lung 183, 186, 190 tuberculosis 214 dissection 338, 339, 346 pancreatic 11 Advanced dynamic flow (ADF) sonographic -

The Supine Pneumothorax
Annals of the Royal College of Surgeons of England (1987) vol. 69 The supine pneumothorax DAVID A P COOKE FRCS Surgical Registrar, Department ofSurgery, St Thomas' Hospital JULIE C COOKE FRCR* Radiological Senior Registrar, Department ofDiagnostic Radiology, Brompton Hospital, London Key words: PNEUMOTHORAX; COMPUTI ED TOMOGRAPHY; TRAUMA Summary TABLE I Causes of a pneumothorax The consequences of an undiagnosed pneumothorax can be life- threatening, particularly in patients with trauma to the head or Broncho-pulmonay pathology Traumatic injuy and in those mechanical ventilation. Yet multiple requiring Asthma it is these patients, whose films will be assessed initially by the Bronchial adenoma surgeon, who are more likely to have a chest X-ray taken in the Bronchial carcinoma Penetrating trauma supine position. The features of supine pneumothoraces are de- Emphysema Blunt trauma scribed and discussed together with radiological techniques used to Fibrosing alveolitis Inhaled foreign body confirm the diagnosis, including computed tomography (CT) Idiopathic which may be ofparticular importance in patients with associated Marfan's syndrome fatrogenic cranial trauma. Pulmonary abscess Pulmonary dysplasia CVP line insertion Introduction Pulmonary infarct Jet ventilation Pulmonary metastases Liver biopsy In a seriously ill patient or the victim of multiple trauma Pulmonoalveolar proteinosis Lung biopsy the clinical symptoms and signs of a pneumothorax may Radiation pneumonitis Oesophageal instrumentation be overshadowed by other problems. Usually in these Sarcoid PEEP ventilation circumstances a chest X-ray will be taken at the bedside Staphylococcal septicaemia Pleural aspiration with the patient supine and the appearances of a Tuberculosis Pleural biopsy pneumothorax will be different from those seen when the Tuberose sclerosis patient is upright. -

Toxicological Profile for Jp-5, Jp-8, and Jet a Fuels
TOXICOLOGICAL PROFILE FOR JP-5, JP-8, AND JET A FUELS U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service Agency for Toxic Substances and Disease Registry March 2017 JP-5, JP-8, AND JET A FUELS ii DISCLAIMER Use of trade names is for identification only and does not imply endorsement by the Agency for Toxic Substances and Disease Registry, the Public Health Service, or the U.S. Department of Health and Human Services. JP-5, JP-8, AND JET A FUELS iii UPDATE STATEMENT A Toxicological Profile for JP-5, JP-8, and Jet A Fuels, Draft for Public Comment was released in February 2016. This edition supersedes any previously released draft or final profile. Toxicological profiles are revised and republished as necessary. For information regarding the update status of previously released profiles, contact ATSDR at: Agency for Toxic Substances and Disease Registry Division of Toxicology and Human Health Sciences Environmental Toxicology Branch 1600 Clifton Road NE Mailstop F-57 Atlanta, Georgia 30329-4027 JP-5, JP-8, AND JET A FUELS iv This page is intentionally blank. JP-5, JP-8, AND JET A FUELS v FOREWORD This toxicological profile is prepared in accordance with guidelines* developed by the Agency for Toxic Substances and Disease Registry (ATSDR) and the Environmental Protection Agency (EPA). The original guidelines were published in the Federal Register on April 17, 1987. Each profile will be revised and republished as necessary. The ATSDR toxicological profile succinctly characterizes the toxicologic and adverse health effects information for these toxic substances described therein. Each peer-reviewed profile identifies and reviews the key literature that describes a substance's toxicologic properties. -

CHEST RADIOLOGY: Goals and Objectives
Harlem Hospital Center Department of Radiology Residency Training Program CHEST RADIOLOGY: Goals and Objectives ROTATION 1 (Radiology Years 1): Resident responsibilities: • ED chest CTs • Inpatient and outpatient plain films including the portable intensive care unit radiographs • Consultations with referring clinicians MEDICAL KNOWLEDGE: • Residents must demonstrate knowledge about established and evolving biomedical, clinical, and cognitive sciences and the application of this knowledge to patient care. At the end of the rotation, the resident should be able to: • Identify normal radiographic and CT anatomy of the chest • Identify and describe common variants of normal, including aging changes. • Demonstrate a basic knowledge of radiographic interpretation of atelectasis, pulmonary infection, congestive heart failure, pleural effusion and common neoplastic diseases of the chest • Identify the common radiologic manifestation of thoracic trauma, including widened mediastinum, signs of aortic laceration, pulmonary contusion/laceration, esophageal and diaphragmatic rupture. • Know the expected postoperative appearance in patients s/p thoracic surgery and the expected location of the life support and monitoring devices on chest radiographs of critically ill patients (intensive care radiology); be able to recognize malpositioned devices. • Identify cardiac enlargement and know the radiographic appearance of the dilated right vs. left atria and right vs. left ventricles, and pulmonary vascular congestion • Recognize common life-threatening -

成人のstridorへの アプローチ 筑波大学総合診療グループ 細井崇弘 監修 筑波大学総合診療グループ 五十野博基 2017年3月9日
成人のstridorへの アプローチ 筑波大学総合診療グループ 細井崇弘 監修 筑波大学総合診療グループ 五十野博基 2017年3月9日 分野:呼吸器 テーマ:診断 症例78歳女性 主訴 呼吸困難 当院は医療過疎地にある100床程度の小規模病院。 【現病歴】高血圧、糖尿病で近医通院中。入院数日前から咳嗽がみら れていた。入院当日に近医を受診し、胸部レントゲン検査を受け肺炎 の疑いと診断され帰宅した。 20時近くまでは同居の娘が異常が無い事を確認。 24時頃に娘が帰宅すると、獣の鳴くような声が聞こえ、患者のもとに 駆け付けると呼吸苦を訴えており、救急車を要請し当院へ搬送された。 【既往歴】高血圧、糖尿病。喘息無し。 【喫煙歴】なし 入院時現症 来院時、自発開眼しているが、会話困難。 Vital sign:BP 145/90mmHg, HR 130/min, RR36/min,SpO2 86%, BT 38℃ 頸部 Stridorを聴取 BVMによる呼吸補助開始するも換気不良 上気道閉塞と判断し、7mmチューブで経口気管挿管 このとき喉頭蓋の浮腫(-) BGA(Vein):pH 7.08, PaO2 63Torr, PCO2 99.9 Torr 胸部所見では両側に軽度の coarse cracklesあり 腹部所見は異常なし 症例の経過 • インフルエンザA(+) • 呼吸器管理となり入院、ラピアクタ投与と鎮痛・鎮静 • CO2ナルコーシスは改善、耳鼻科と共に喉頭ファイバーで声門より 上部に狭窄病変が無い事を確認。 • 意識レベルも清明であり従命可能、自発呼吸も良好であり抜管 抜管後は発語もあり問題なかったが、10数分で呼吸困難を訴え、 SpO2が急激に低下したため再挿管。 再挿管時、声帯は確認できたがチューブが声帯を越えるのに非常に 難渋した。 Stridorは何が原因だったのか? 抜管による喉頭浮腫? では救急外来に来た際の最初のStridorは? Stridorが聞こえたが喉頭蓋炎ではなかったとき、 どのような鑑別疾患を上げ、 どのようにアプローチするのが良かったのか? Clinical Ques3on • 成人で気道狭窄が疑われる場合 ①鑑別診断は何か? ②初期診断のためのアプローチは? Stridorとは~身体診察~ • 強い楽音様の音で、明確な一定の音調(一般に400Hz前後)であり、 通過障害(閉塞)を示唆する。 ・Stridorは吸気時に限られる ・Stridorは常に頸部でより強く聴取されるが、wheezingは常に胸部で 聴取される。Am Rev Respir Dis.1983;143:890-892 ・気道の直径が8㎜以下になると労作時呼吸困難が出現し、 Crit Care Med 2004; 169: 1278-1297. ・気道の直系が5mm以下で安静時にも呼吸苦が出現する。 JAMA.1971;216:1984-1985 気道狭窄の分類 upper airway obstrucYon(UAO) Central airway obstrucYon(CAO) Lower airway obstrucYon(LAO) 口から(鼻腔含め)喉頭. 慢性的な閉塞性肺傷害、喘息や : :気管および主気管支 : CAOと同時に認められることも COPDなど。CAOやUAOとは典型 的には合併しない。 発症率などの疫学については、 (悪性腫瘍以外の例では) 成人での発生頻度が非常にまれであり、 詳しくは分かっていない。 Up to date: Clinical presentaon , diagnosYc evaluaon,and management of -

CT Signs in the Lungs Girish S
CT Signs in the Lungs Girish S. Shroff, MD,* Edith M. Marom, MD,† Myrna C.B. Godoy, MD, PhD,* Mylene T. Truong, MD,* and Caroline Chiles, MDz Radiologic signs are often based on items or patterns that are encountered in everyday life. They are especially useful because their observation allows the differential diagnosis to be narrowed, and in some cases, enables a diagnosis to be made. In this review, several clas- sic and newer computed tomography signs in the lungs are discussed. Semin Ultrasound CT MRI 40:265-274 © 2018 Elsevier Inc. All rights reserved. Introduction should be considered when the pipe cleaner sign is seen (Fig. 2). Nodule distribution varies slightly among the con- adiologic signs are often based on items or patterns that ditions—in sarcoidosis, nodules tend to predominate along R are encountered in everyday life. They are especially use- larger bronchovascular bundles and in the subpleural ful because their observation allows the differential diagnosis regions whereas in silicosis and coal worker’s pneumoconi- to be narrowed, and in some cases, enables a diagnosis to be osis, nodules tend to predominate in the centrilobular and made. Furthermore, early recognition of signs associated subpleural regions.2 Smooth or nodular interlobular septal with aggressive infections may be life-saving. In this review, thickening is usually the dominant feature in lymphangitic the following computed tomography (CT) signs in the lungs carcinomatosis. Interlobular septal thickening is typically will be discussed: pipe cleaner, halo, reversed halo, air cres- absent in granulomatous diseases such as sarcoidosis and cent, Monod, Cheerio, straight edge, air bronchogram, tree- silicosis.