Traumatic Aniridia and Aphakia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Novel Yamane Technique Modification for Haptic Exposure After Glued Intrascleral Haptic Fixation
American Journal of Ophthalmology Case Reports 14 (2019) 101–104 Contents lists available at ScienceDirect American Journal of Ophthalmology Case Reports journal homepage: www.elsevier.com/locate/ajoc Case report Novel yamane technique modification for haptic exposure after glued intrascleral haptic fixation T ∗ Rachel A. Gelman, Sumit Garg Gavin Herbert Eye Institute, University of California, Irvine, 92697, United States ARTICLE INFO ABSTRACT Keywords: The field of intraocular lens fixation in the setting of inadequate capsular support is a dynamic one as surgical Yamane technique approaches are constantly evolving. There has been a paradigm shift towards the use of sutureless methods of Haptic exposure scleral fixation to avoid suture-related complications. In the latest described style of scleral fixation, IOLs can be Intraocular lens (IOL) secured without suture or “glue”, and rather with the creation of a flange on each haptic that allows for firm intrascleral fixation. We describe a modification of the flange technique to refixate patients with glued IOLs who developed haptic extrusion and required surgical intervention. 1. Introduction 48% of eyes with a sutured IOL. The risk of postoperative glaucoma was also found to be lower, occurring 16% of the time with glued IOLs 1.1. Sutureless techniques for scleral fixation versus 40% with sutured IOLs.5 Furthermore, the use of glue over su- ture precludes problems such as suture breakage and exposure, which In 2007, Agarwal et al. described the use of fibrin glue for fixation of in a retrospective study by McAllister et al. occurred at a rate of 6.1% a posterior chamber IOL in the setting of deficient capsular support in a and 11% respectively with the use of 10–0 polypropylene.2 Degradation technique referred to as “glued IOL fixation”. -

Infantile Aphakia and Successful Fitting of Pediatric Contact Lenses; a Case Presentation Authors: Virji N, Patel A, Libassi D
Infantile aphakia and successful fitting of pediatric contact lenses; a case presentation Authors: Virji N, Patel A, Libassi D An eleven month old male presents with bilateral aphakia secondary to congenital cataracts. The patient is currently successfully wearing B&L Silsoft Pediatric contact lenses, with good prognosis for vision in both eyes. I. Case History -Patient demographics: African American male, DOB 8/18/2009 -Chief complaint: patient presents with bilateral aphakia secondary to bilateral congenital cataract extraction -Ocular, medical history: S/P CE with anterior vitrectomy OD 09/22/2009, followed by OS 09/29/09. (+) squinting, rubs eyes, light sensitivity -Medications: none -Other salient information: patient has been seen by SUNY Contact Lens clinic since 2 months old, 10/14/2009 II. Pertinent findings -Clinical: Keratometry readings 41.00/41.25 @ 005 OD, 38.50/41.00 @ 046 Axial length, immeasurable Horizontal corneal diameter 8mm OD/OS Fundus exam WNL OU -Others: surgical dates: successful CE OU, September 2009 III. Differential diagnosis -Primary/leading: Idiopathic -Others: Posterior lenticonus, persistent hyperplastic primary vitreous, anterior segment dysgenesis, and posterior pole tumors, trauma, intrauterine infection (rubella), maternal hypoglycemia, trisomy (eg, Down, Edward, and Patau syndromes), myotonic dystrophy, infectious diseases (eg, toxoplasmosis, rubella, cytomegalovirus, and herpes simplex [TORCH]), and prematurity. (5) IV. Diagnosis and discussion -Elaborate on the condition: Bilateral infantile cataracts are one of the major treatable causes of visual impairment in children. (2) Hubel and Weisel’s research on the critical period of visual development determined that if infantile cataracts are removed within the critical period and appropriate correction is worn, vision is greatly improved. -

Posterior Chamber Scleral Fixation of Intraocular Lenses in Post
DOI: 10.7860/JCDR/2017/20989.9533 Original Article Posterior Chamber Scleral Fixation of Intraocular Lenses in Post- Ophthalmology Section Vitrectomised Aphakic Eyes FRANCIS KWASI OBENG1, VIPAN KUMAR VIG2, PREETAM SINGH3, RAJBIR SINGH4, BODHRAJ DHAWAN5, NIKHIL SAHAJPAL6 ABSTRACT Materials and Methods: Records of all patients who had Introduction: The best method of aphakia correction is in the undergone secondary PCSFIOL implantation with sutures bag implantation of Posterior Chamber Intraocular Lens (PCIOL). after combined PPV and lensectomy from 2010 to 2014 were When this ideal procedure is not possible due to lack of integrity reviewed retrospectively for visual outcomes and complications. of posterior capsule or zonules, the other alternatives are broadly Patients’ demographic data, indication for PPV, best corrected categorized into two: extraocular and intraocular. Whereas, the preoperative and postoperative visual acuities, complications former includes contact lenses and aphakic glasses, the latter of surgery, and indications of PCSFIOL and length of follow up ones are further divided into anterior and posterior chamber were collected and analyzed. methods. Anterior Chamber Intraocular Lenses (ACIOL) can be Results: A total of 148 eyes of 148 patients (127 males and with or without iris claw. At the posterior chamber, fixation of the 21 females) were identified. Mean age at surgery was 32.5+8 lenses can be with glue or sutures. years (range 2.5-73 years) with a mean follow up 23+14 months When there is combined Pars Plana Vitrectomy (PPV) and (range 3-114 months). A total of 95.27%, 2.70% and 2.02% lensectomy or if the indication of PPV is dropped nucleus or of patients had improvement, maintenance and worsening of intraocular lens, a modality of aphakia correction should be their final postoperative visual acuities respectively. -

Intraocular Lenses and Spectacle Correction
MEDICAL POLICY POLICY TITLE INTRAOCULAR LENSES, SPECTACLE CORRECTION AND IRIS PROSTHESIS POLICY NUMBER MP-6.058 Original Issue Date (Created): 6/2/2020 Most Recent Review Date (Revised): 6/9/2020 Effective Date: 2/1/2021 POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS DISCLAIMER CODING INFORMATION REFERENCES POLICY HISTORY I. POLICY Intraocular Lens Implant (IOL) Initial IOL Implant A standard monofocal intraocular lens (IOL) implant is medically necessary when the eye’s natural lens is absent including the following: Following cataract extraction Trauma to the eye which has damaged the lens Congenital cataract Congenital aphakia Lens subluxation/displacement A standard monofocal intraocular lens (IOL) implant is medically necessary for anisometropia of 3 diopters or greater, and uncorrectable vision with the use of glasses or contact lenses. Premium intraocular lens implants including but not limited to the following are not medically necessary for any indication, including aphakia, because each is intended to reduce the need for reading glasses. Presbyopia correcting IOL (e.g., Array® Model SA40, ReZoom™, AcrySof® ReStor®, TECNIS® Multifocal IOL, Tecnis Symfony and Tecnis SymfonyToric, TRULIGN, Toric IO, Crystalens Aspheric Optic™) Astigmatism correcting IOL (e.g., AcrySof IQ Toric IOL (Alcon) and Tecnis Toric Aspheric IOL) Phakic IOL (e.g., ARTISAN®, STAAR Visian ICL™) Replacement IOLs MEDICAL POLICY POLICY TITLE INTRAOCULAR LENSES, SPECTACLE CORRECTION AND IRIS PROSTHESIS POLICY NUMBER -

Expanding the Phenotypic Spectrum of PAX6 Mutations: from Congenital Cataracts to Nystagmus
G C A T T A C G G C A T genes Article Expanding the Phenotypic Spectrum of PAX6 Mutations: From Congenital Cataracts to Nystagmus Maria Nieves-Moreno 1,* , Susana Noval 1 , Jesus Peralta 1, María Palomares-Bralo 2 , Angela del Pozo 3 , Sixto Garcia-Miñaur 4, Fernando Santos-Simarro 4 and Elena Vallespin 5 1 Department of Ophthalmology, Hospital Universitario La Paz, 28046 Madrid, Spain; [email protected] (S.N.); [email protected] (J.P.) 2 Department of Molecular Developmental Disorders, Medical and Molecular Genetics Institue (INGEMM) IdiPaz, CIBERER, Hospital Universitario La Paz, 28046 Madrid, Spain; [email protected] 3 Department of Bioinformatics, Medical and Molecular Genetics Institue (INGEMM) IdiPaz, CIBERER, Hospital Universitario La Paz, 28046 Madrid, Spain; [email protected] 4 Department of Clinical Genetics, Medical and Molecular Genetics Institue (INGEMM) IdiPaz, CIBERER, Hospital Universitario La Paz, 28046 Madrid, Spain; [email protected] (S.G.-M.); [email protected] (F.S.-S.) 5 Department of Molecular Ophthalmology, Medical and Molecular Genetics Institue (INGEMM) IdiPaz, CIBERER, Hospital Universitario La Paz, 28046 Madrid, Spain; [email protected] * Correspondence: [email protected] Abstract: Background: Congenital aniridia is a complex ocular disorder, usually associated with severe visual impairment, generally caused by mutations on the PAX6 gene. The clinical phenotype of PAX6 mutations is highly variable, making the genotype–phenotype correlations difficult to establish. Methods: we describe the phenotype of eight patients from seven unrelated families Citation: Nieves-Moreno, M.; Noval, with confirmed mutations in PAX6, and very different clinical manifestations. -
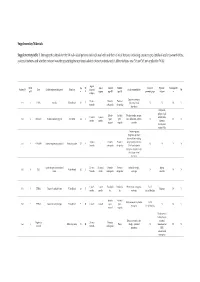
Solved/Unsolved
Supplementary Materials: Supplementary table 1. Demographic details for the 54 individual patients (solved/unsolved) and their clinical features including cataract type, details of ocular co-morbidities, systemic features and whether cataract was the presenting feature (non-isolated cataract patients only). Abbreviations: yes (Y), no (N), not applicable (N/A). Age at Famil Ag M/ Age at Cataract Cataract Cataract Systemic Consanguinit Patient ID Gene Confirmed genetic diagnosis Ethnicity diagnosi Ocular co-morbidities FH y ID e F surgery type RE type LE presenting sign features y s (days) Aniridia, nystagmus, 23 years Posterior Posterior 1-1 1 PAX6 Aniridia White British 25 F - glaucoma, foveal N N N Y 4 months subcapsular subcapsular hypoplasia Cleft palate, epilepsy, high Aphakia Aphakia Macular atrophy, myopia, 7 years 9 7 years 8 arched palate, 2-1 2 COL11A1 Stickler syndrome, type II Not Stated 34 F (post- (post- lens subluxation, vitreous N N N months months flattened surgical) surgical) anomaly maxilla, short stature (5'2ft) Anterior segment dysgenesis, pupillary abnormalities including 12 years Posterior Posterior ectopic pupils, ectropion 3-1 3 CPAMD8 Anterior segment dysgenesis 8 Other, Any other 27 F - N N Y N 5 months subcapsular subcapsular UVAE and irodensis, nystagmus, dysplastic optic discs, large corneal diameters Gyrate atrophy of choroid and 23 years 29 years 1 Posterior Posterior Retinal dystrophy, Bipolar 4-1 4 OAT White British 42 F N N N retina 7 months month subcapsular subcapsular exotropia disorder 1 year 6 1 year -

Feasibility and Outcome of Descemet Membrane Endothelial Keratoplasty in Complex Anterior Segment and Vitreous Disease
CLINICAL SCIENCE Feasibility and Outcome of Descemet Membrane Endothelial Keratoplasty in Complex Anterior Segment and Vitreous Disease Julia M. Weller, MD, Theofilos Tourtas, MD, and Friedrich E. Kruse, MD escemet membrane endothelial keratoplasty (DMEK), Purpose: Descemet membrane endothelial keratoplasty (DMEK) is Da technique for posterior lamellar keratoplasty, involves becoming the method of choice for treating Fuchs endothelial a graft consisting only of the thin Descemet membrane with dystrophy and pseudophakic bullous keratopathy. We investigated adherent corneal endothelial cells. Introduced in 2006 by whether DMEK can serve as a routine procedure in endothelial Melles et al,1 DMEK is becoming more popular as several decompensation even in complex preoperative situations. studies show its superiority to Descemet stripping automated Methods: Of a total of 1184 DMEK surgeries, 24 consecutive eyes endothelial keratoplasty (DSAEK), regarding visual function 2,3 with endothelial decompensation and complex preoperative situa- and the time of visual rehabilitation after DMEK. However, tions were retrospectively analyzed and divided into 5 groups: group because DMEK grafts are thinner than DSAEK grafts, it is fi 1: irido-corneo-endothelial syndrome (n = 3), group 2: aphakia, more dif cult to handle them and typically takes surgeons subluxated posterior chamber intraocular lens or anterior chamber longer to learn. intraocular lens (n = 6), group 3: DMEK after trabeculectomy (n = In difficult situations, most surgeons prefer DSAEK or 4), group 4: DMEK with simultaneous intravitreal injection (n = 6), penetrating keratoplasty to DMEK because of its possible and group 5: DMEK after vitrectomy (n = 5). Main outcome intraoperative complications. For example, if corneal edema 4 parameters were best-corrected visual acuity, central corneal thick- is advanced, Ham et al recommend performing DSAEK first ness, endothelial cell density, rebubbling rate, and graft failure rate. -

Glaucoma-Related Adverse Events in the Infant Aphakia Treatment Study 1-Year Results
CLINICAL SCIENCES ONLINE FIRST Glaucoma-Related Adverse Events in the Infant Aphakia Treatment Study 1-Year Results Allen D. Beck, MD; Sharon F. Freedman, MD; Michael J. Lynn, MS; Erick Bothun, MD; Daniel E. Neely, MD; Scott R. Lambert, MD; for the Infant Aphakia Treatment Study Group Objectives: To report the incidence of glaucoma and glau- sistent fetal vasculature and 1.6 times higher for each coma suspects in the IATS, and to evaluate risk factors for month of age younger at cataract surgery. the development of a glaucoma-related adverse event in patients in the IATS in the first year of follow-up. Conclusions: Modern surgical techniques do not elimi- nate the early development of glaucoma following con- Methods: A total of 114 infants between 1 and 6 months genital cataract surgery with or without an intraocular of age with a unilateral congenital cataract were as- lens implant. Younger patients with or without persis- signed to undergo cataract surgery either with or with- tent fetal vasculature seem more likely to develop a glau- out an intraocular lens implant. Standardized defini- coma-related adverse event in the first year of follow- tions of glaucoma and glaucoma suspect were created and up. Vigilance for the early development of glaucoma is used in the IATS. needed following congenital cataract surgery, especially when surgery is performed during early infancy or for a Results: Of these 114 patients, 10 (9%) developed glau- child with persistent fetal vasculature. Five-year fol- coma and 4 (4%) had glaucoma suspect, for a total of 14 low-up data for the IATS will likely reveal more glaucoma- patients (12%) with a glaucoma-related adverse event in related adverse events. -

Journal of Ophthalmology & Clinical Research
ISSN: 2573-9573 Case Report Journal of Ophthalmology & Clinical Research Bilateral Congenital Ectropion Uveae, Anterior Segment Dysgenesis and Aniridia with Microspherophakic Congenital Cataracts and RubeosisIridis Rao Muhammad Arif Khan* and Ashal Kaiser Pal *Corresponding author Rao Muhammad Arif Khan, MCPS, FCPS, FPO, FACS, Pediatric Ophthalmologist, King Edward Medical University, Al-Awali Street, Taif Road, Makkah, Saudi Arabia, Pediatric Ophthalmologist, King Edward Medical University, Tel: 00966560479694; E-mail: [email protected] Makkah, Saudi Arabia Submitted: 02 Apr 2018; Accepted: 12 Apr 2018; Published: 19 Apr 2018 Abstract In recent times, multiple eye diseases have been seen associated with an increase in the rate of Demodex infestation as a possible cause, but in the particular case of dry eye syndrome in patients treated with platelet-rich plasma, this increase in mite may be relevant to guide a more adequate treatment focusing on the elimination of the mite in conjunction with the recovery of the ocular ecology. The demodex mite is a commensal parasite that lives in hair follicles, sebaceous glands and meibomian, which in a high rate of infestation can generate alterations in the ocular area. Performing an adequate diagnosis for the detection of the mite and treatment for its eradication can be effective for the recovery of the normal physiology of the tear film that constitutes a cause of dry eye. Introduction Congenital ectropion uvea is a rare ocular manifestation of neural crest syndrome [1]. It is a non-progressive anomaly characterized by presence of iris pigment epithelium on anterior surface of iris from the pigment ruff [2]. Congenital glaucoma is its common association [3-8]. -

Congenital Ocular Anomalies in Newborns: a Practical Atlas
www.jpnim.com Open Access eISSN: 2281-0692 Journal of Pediatric and Neonatal Individualized Medicine 2020;9(2):e090207 doi: 10.7363/090207 Received: 2019 Jul 19; revised: 2019 Jul 23; accepted: 2019 Jul 24; published online: 2020 Sept 04 Mini Atlas Congenital ocular anomalies in newborns: a practical atlas Federico Mecarini1, Vassilios Fanos1,2, Giangiorgio Crisponi1 1Neonatal Intensive Care Unit, Azienda Ospedaliero-Universitaria Cagliari, University of Cagliari, Cagliari, Italy 2Department of Surgery, University of Cagliari, Cagliari, Italy Abstract All newborns should be examined for ocular structural abnormalities, an essential part of the newborn assessment. Early detection of congenital ocular disorders is important to begin appropriate medical or surgical therapy and to prevent visual problems and blindness, which could deeply affect a child’s life. The present review aims to describe the main congenital ocular anomalies in newborns and provide images in order to help the physician in current clinical practice. Keywords Congenital ocular anomalies, newborn, anophthalmia, microphthalmia, aniridia, iris coloboma, glaucoma, blepharoptosis, epibulbar dermoids, eyelid haemangioma, hypertelorism, hypotelorism, ankyloblepharon filiforme adnatum, dacryocystitis, dacryostenosis, blepharophimosis, chemosis, blue sclera, corneal opacity. Corresponding author Federico Mecarini, MD, Neonatal Intensive Care Unit, Azienda Ospedaliero-Universitaria Cagliari, University of Cagliari, Cagliari, Italy; tel.: (+39) 3298343193; e-mail: [email protected]. -

Visual Management of Aphakia with Concomitant Severe Corneal Irregularity by Mini-Scleral Design Contact Lenses
HOSTED BY Available online at www.sciencedirect.com ScienceDirect Journal of Current Ophthalmology 28 (2016) 27e31 http://www.journals.elsevier.com/journal-of-current-ophthalmology Original research Visual management of aphakia with concomitant severe corneal irregularity by mini-scleral design contact lenses Fateme Alipur, Seyedeh Simindokht Hosseini* Eye Research Center, Farabi Eye Hospital, Tehran University of Medical Sciences, Tehran, Iran Received 30 October 2015; accepted 28 January 2016 Available online 30 March 2016 Abstract Purpose: To evaluate visual results, comfort of use, safety, and efficacy of mini scleral contact lenses in optical management in patients with traumatic aphakia and severe concomitant irido-corneal injury. Methods: In a case series, eight eyes with post traumatic aphakia and severe concomitant irido-corneal injury that were evaluated at the Contact Lens Clinic of Farabi Eye Hospital, Tehran, Iran for contact lens fitting and could not be corrected with conventional corneal RGP contact lenses were fitted with miniscleral contact lenses. Uncorrected visual acuity (UCVA), best spectacle corrected visual acuity (BSCVA), and BCVA (Best corrected visual acuity) with miniscleral lens were recorded. Slit lamp examination, comfortable daily wearing time, and any contact lens-related complication were documented in each follow-up visit. Results: The mean UCVA and BSCVA of the cases was >2.7 and 0.41 LogMAR, respectively (BSCVA could not be assessed in one case due to severe corneal irregularity). The mean final BCVA with the miniscleral lens was 0.05 LogMAR (range from 0.4 to À0.04 LogMAR). The mean follow-up period was 14.6 months. The mean comfortable daily wearing time (CDWT) was 11.6 h, ranging from 8 to 16 h. -

Eleventh Edition
SUPPLEMENT TO April 15, 2009 A JOBSON PUBLICATION www.revoptom.com Eleventh Edition Joseph W. Sowka, O.D., FAAO, Dipl. Andrew S. Gurwood, O.D., FAAO, Dipl. Alan G. Kabat, O.D., FAAO Supported by an unrestricted grant from Alcon, Inc. 001_ro0409_handbook 4/2/09 9:42 AM Page 4 TABLE OF CONTENTS Eyelids & Adnexa Conjunctiva & Sclera Cornea Uvea & Glaucoma Viitreous & Retiina Neuro-Ophthalmic Disease Oculosystemic Disease EYELIDS & ADNEXA VITREOUS & RETINA Blow-Out Fracture................................................ 6 Asteroid Hyalosis ................................................33 Acquired Ptosis ................................................... 7 Retinal Arterial Macroaneurysm............................34 Acquired Entropion ............................................. 9 Retinal Emboli.....................................................36 Verruca & Papilloma............................................11 Hypertensive Retinopathy.....................................37 Idiopathic Juxtafoveal Retinal Telangiectasia...........39 CONJUNCTIVA & SCLERA Ocular Ischemic Syndrome...................................40 Scleral Melt ........................................................13 Retinal Artery Occlusion ......................................42 Giant Papillary Conjunctivitis................................14 Conjunctival Lymphoma .......................................15 NEURO-OPHTHALMIC DISEASE Blue Sclera .........................................................17 Dorsal Midbrain Syndrome ..................................45