Intraocular Lenses and Spectacle Correction
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Stereo Capture and Display At
Creation of a Complete Stereoscopic 3D Workflow for SoFA Allison Hettinger and Ian Krassner 1. Introduction 1.1 3D Trend Stereoscopic 3D motion pictures have recently risen to popularity once again following the success of films such as James Cameron’s Avatar. More and more films are being converted to 3D but few films are being shot in 3D. Current available technology and knowledge of that technology (along with cost) is preventing most films from being shot in 3D. Shooting in 3D is an advantage because two slightly different images are produced that mimic the two images the eyes see in normal vision. Many take the cheaper route of shooting in 2D and converting to 3D. This results in a 3D image, but usually nowhere near the quality as if the film was originally shot in 3D. This is because a computer has to create the second image, which can result in errors. It is also important to note that a 3D image does not necessarily mean a stereo image. 3D can be used to describe images that have an appearance of depth, such as 3D animations. Stereo images refer to images that make use of retinal disparity to create the illusion of objects going out of and into the screen plane. Stereo images are optical illusions that make use of several cues that the brain uses to perceive a scene. Examples of monocular cues are relative size and position, texture gradient, perspective and occlusion. These cues help us determine the relative depth positions of objects in an image. Binocular cues such as retinal disparity and convergence are what give the illusion of depth. -

A Virtual Try-On System for Prescription Eyeglasses
Feature Article A Virtual Try-On System for Prescription Eyeglasses Qian Zhang and Yu Guo ■ Nanyang Technological University Pierre-Yves Laffont, Tobias Martin, and Markus Gross ■ ETH Zurich ision-correcting eyeglasses have improved We present a system for virtually trying on pre- the lives of millions of people. Eyeglasses scription eyeglasses. Our system acts as a virtual significantly affect the wearer’s appear- mirror, allowing users to try on a variety of eye- Vance, and the selection of new pairs of eyeglasses is glasses with corrective lenses based on their pre- largely based on how the glasses look when wearers scription (see Figure 2). We use an image sequence try them on. However, an often overlooked fact is of the user without eyeglasses as input, along with that corrective lenses introduce distortion caused the user’s eyeglasses prescription and a 3D model by the refraction effect. As Figure 1 illustrates, the of the desired eyeglasses frame. Our system gen- eyes of a person wearing correc- erates a 3D representation of the corrective lenses tive lenses for nearsightedness mounted into the eyeglasses frame and modifies the Corrective lenses introduce appear smaller compared with video sequence to virtually insert the eyeglasses us- distortion caused by the wearing nonprescription lenses, ing image-based rendering. This approach simulates refraction effect, which whereas the eyes of a person the distortion introduced by the prescription lenses changes the wearer’s wearing lenses for farsightedness and gives users a better idea of how they would look appearance. To give users a appear larger. when wearing the new pair of eyeglasses. -

Prescription Companion
PRESCRIPTION COMPANION ©2012Transitions Optical inc. ophthalmic lens technical reference JUBILEE YEAR 2012 E -Edition 7 www.norville.co.uk Introduction and Page Index The Norville Companion is a supporting publication for our Prescription Catalogue, providing further technical details, hints and ideas gleaned from everyday experiences. TOPIC Page(s) TOPIC Page(s) Index 2 - 3 Part II Rx Allsorts Lens Shapes 4 - 6 Lens Forms 49 Effective Diameter Chart 7 Base Curves 50 - 51 Simplify Rx 8 Aspherics 52 - 53 Ophthalmic Resins 9 Free-form Digital Design 54 Indices of Ophthalmic lenses - Resin 10 Compensated Lens Powers 55 - 56 Polycarbonate 11 Intelligent Prism Thinning 57 - 58 Trivex 12 - 13 Superlenti - Glass 59 Resin Photochromic Lenses 14 Superlenti - Resin 60 Transitions Availability Check List 15 V Value / Fresnels 61 Nupolar Polarising Lenses 16 E Style Bifocal / Trifocal 62 Drivewear Lenses 17 - 18 Photochromic / Glazing / Prisms 63 UV Protective Lenses 19 Lens Measures 64 Norville PLS Tints 20 Sports 65 Tinted Resin Lenses 21 3D Technology Overview 66 Mid and High Index Resins Tintability 22 Rx Ordering 67 Norlite Tint Transmission Charts 23 - 25 Order Progress 68 Norlite Speciality Tinted Resins 26 - 31 Rx Order Form 69 Norlite Mirror Coating 32 Queries 70 Reflection Free Coating 33 - 34 Optical Heritage 71 F.A.Q. Reflection Free Coatings 35 - 37 Rx House - Change afoot? 72 - 73 Indices of Ophthalmic Lenses - Glass 38 Remote Edging 74 Glass Photochromic Lenses 38 Remote edging - F.A.Q. 75 Speciality Absorbing Glass 39 Quality Assurance -

The Eye Is a Natural Optical Tool
KEY CONCEPT The eye is a natural optical tool. BEFORE, you learned NOW, you will learn •Mirrors and lenses focus light • How the eye depends on to form images natural lenses •Mirrors and lenses can alter • How artificial lenses can be images in useful ways used to correct vision problems VOCABULARY EXPLORE Focusing Vision cornea p. 607 How does the eye focus an image? pupil p. 607 retina p. 607 PROCEDURE 1 Position yourself so you can see an object about 6 meters (20 feet) away. 2 Close one eye, hold up your index finger, and bring it as close to your open eye as you can while keeping the finger clearly in focus. 3 Keeping your finger in place, look just to the side at the more distant object and focus your eye on it. 4 Without looking away from the more distant object, observe your finger. WHAT DO YOU THINK? • How does the nearby object look when you are focusing on something distant? • What might be happening in your eye to cause this change in the nearby object? The eye gathers and focuses light. The eyes of human beings and many other animals are natural optical tools that process visible light. Eyes transmit light, refract light, and respond to different wavelengths of light. Eyes contain natural lenses that focus images of objects. Eyes convert the energy of light waves into signals that can be sent to the brain. The brain interprets these signals as shape, brightness, and color. Altogether, these processes make vision possible. In this section, you will learn how the eye works. -

Infantile Aphakia and Successful Fitting of Pediatric Contact Lenses; a Case Presentation Authors: Virji N, Patel A, Libassi D
Infantile aphakia and successful fitting of pediatric contact lenses; a case presentation Authors: Virji N, Patel A, Libassi D An eleven month old male presents with bilateral aphakia secondary to congenital cataracts. The patient is currently successfully wearing B&L Silsoft Pediatric contact lenses, with good prognosis for vision in both eyes. I. Case History -Patient demographics: African American male, DOB 8/18/2009 -Chief complaint: patient presents with bilateral aphakia secondary to bilateral congenital cataract extraction -Ocular, medical history: S/P CE with anterior vitrectomy OD 09/22/2009, followed by OS 09/29/09. (+) squinting, rubs eyes, light sensitivity -Medications: none -Other salient information: patient has been seen by SUNY Contact Lens clinic since 2 months old, 10/14/2009 II. Pertinent findings -Clinical: Keratometry readings 41.00/41.25 @ 005 OD, 38.50/41.00 @ 046 Axial length, immeasurable Horizontal corneal diameter 8mm OD/OS Fundus exam WNL OU -Others: surgical dates: successful CE OU, September 2009 III. Differential diagnosis -Primary/leading: Idiopathic -Others: Posterior lenticonus, persistent hyperplastic primary vitreous, anterior segment dysgenesis, and posterior pole tumors, trauma, intrauterine infection (rubella), maternal hypoglycemia, trisomy (eg, Down, Edward, and Patau syndromes), myotonic dystrophy, infectious diseases (eg, toxoplasmosis, rubella, cytomegalovirus, and herpes simplex [TORCH]), and prematurity. (5) IV. Diagnosis and discussion -Elaborate on the condition: Bilateral infantile cataracts are one of the major treatable causes of visual impairment in children. (2) Hubel and Weisel’s research on the critical period of visual development determined that if infantile cataracts are removed within the critical period and appropriate correction is worn, vision is greatly improved. -

Virtual Reality and Visual Perception by Jared Bendis
Virtual Reality and Visual Perception by Jared Bendis Introduction Goldstein (2002) defines perception as a “conscious sensory experience” (p. 6) and as scientists investigate how the human perceptual system works they also find themselves investigating how the human perceptual system doesn’t work and how that system can be fooled, exploited, and even circumvented. The pioneers in the ability to control the human perceptual system have been in the field of Virtual Realty. In Simulated and Virtual Realities – Elements of Perception, Carr (1995) defines Virtual Reality as “…the stimulation of human perceptual experience to create an impression of something which is not really there” (p. 5). Heilig (2001) refers to this form of “realism” as “experience” and in his 1955 article about “The Cinema of the Future” where he addresses the need to look carefully at perception and breaks down the precedence of perceptual attention as: Sight 70% Hearing 20% Smell 5% Touch 4% Taste 1% (p. 247) Not surprisingly sight is considered the most important of the senses as Leonardo da Vinci observed: “They eye deludes itself less than any of the other senses, because it sees by none other than the straight lines which compose a pyramid, the base of which is the object, and the lines conduct the object to the eye… But the ear is strongly subject to delusions about the location and distance of its objects because the images [of sound] do not reach it in straight lines, like those of the eye, but by tortuous and reflexive lines. … The sense of smells is even less able to locate the source of an odour. -

Patient Instruction Guide
1‐DAY ACUVUE® MOIST Brand Contact Lenses 1‐DAY ACUVUE® MOIST Brand Contact Lenses for ASTIGMATISM 1‐DAY ACUVUE® MOIST Brand MULTIFOCAL Contact Lenses etafilcon A Soft (hydrophilic) Contact Lenses Visibility Tinted with UV Blocker for Daily Disposable Wear PATIENT INSTRUCTION GUIDE CAUTION: U.S. Federal law restricts this device to sale by or on the order of a licensed practitioner. 1 TABLE OF CONTENTS TABLE OF CONTENTS ............................................................................................................................................... 2 INTRODUCTION ....................................................................................................................................................... 3 SYMBOLS KEY .......................................................................................................................................................... 4 UNDERSTANDING YOUR PRESCRIPTION ................................................................................................................. 5 GLOSSARY OF COMMONLY USED TERMS ............................................................................................................... 5 WEARING RESTRICTIONS & INDICATIONS ............................................................................................................... 6 WHEN LENSES SHOULD NOT BE WORN (CONTRAINDICATIONS) ............................................................................ 6 WARNINGS ............................................................................................................................................................. -

A Breakthrough in Myopia Control for Your Child
USER GUIDE FOR MiYOSMART MiYOSMART OPTOMETRIC PROTOCOL FOR MiYOSMART MiYOSMART MiYOSMART: A SMART APPROACH TO MYOPIA MiYOSMART The user guide identies what new MiyoSmart wearers should take note of during the adaptation period. To ensure maximized benets of MiyoSmart are experienced, it is recommended to follow the optometric protocol. If you had a way to halt or slow down the progression of myopia, surely you would want to know how. Adaption to new lenses MiyoSmart delivers on this promise and lls a rapidly growing market need. MiyoSmart is an innovative 1ST VISIT 1. It always takes time to get used to your new lenses. The time needed really depends on the individual but wearers can ophthalmic lens for myopia control developed by Hoya Vision Care in cooperation with its research expect about one to two weeks to adapt. collaborator, The Hong Kong Polytechnic University (PolyU). Engineered specically to correct myopic During the rst visit, all visual functions of the child should be assessed to get a clear overview of the current status. A few 2. During the adaptation time, the wearer should avoid: factors are examined to ascertain if the wearer is suitable for MiyoSmart. refractive error and slow down myopia progression, MiyoSmart comes to market at a time when the 1 It is also essential to know the child and his/her parents' ocular and optical history. incidence of myopia is on the rise. Preliminary investigation will also have to be done, where it is compulsory to conduct the following tests and examinations: An estimated 5 billion Research shows that Intensive sport Operating any Using the new lenses Using them on high people, or activities, e.g. -
Contact Lenses
Buying Contact Lenses Some common Questions and Answers to help you buy your lenses safely Wearing contact lenses offers many benefits. Following some simple precautions when buying lenses can help to make sure that you don’t put the health and comfort of your eyes at risk. The British Contact Lens Association and General Optical Council have put together some common questions and answers to help you buy your lenses safely 2 Images courtesy of College of Optometrists, General Optical Council and Optician How do I find out about wearing contact lenses? ● If you want to wear contact lenses to correct your eyesight, you must start by consulting an eye care practitioner for a fitting. Only registered optometrists, dispensing opticians with a specialist qualification (contact lens opticians) and medical practitioners can fit contact lenses. Fitting includes discussing your visual and lifestyle requirements, an eye examination to make sure your eyes are healthy and find out if you’re suitable, and measurements of your eyes to ensure the best lens type, fit and vision, before trying lenses. Once you have worn the lenses, you should have the health of your eyes checked again. You will also need to learn how to handle and care for your lenses. Your practitioner will advise you when you should wear the lenses and how often you should replace them. When is the fitting completed? ● Your prescribing practitioner will tell you when the fitting is completed. How long the fitting takes will depend on your lens type and your eye health. Don’t forget that, once fitted, you will need to have regular check-ups to make sure your eyes are healthy and to get the best from your contact lenses. -
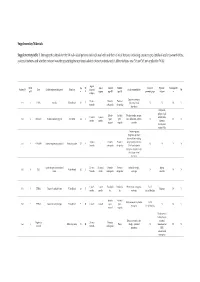
Solved/Unsolved
Supplementary Materials: Supplementary table 1. Demographic details for the 54 individual patients (solved/unsolved) and their clinical features including cataract type, details of ocular co-morbidities, systemic features and whether cataract was the presenting feature (non-isolated cataract patients only). Abbreviations: yes (Y), no (N), not applicable (N/A). Age at Famil Ag M/ Age at Cataract Cataract Cataract Systemic Consanguinit Patient ID Gene Confirmed genetic diagnosis Ethnicity diagnosi Ocular co-morbidities FH y ID e F surgery type RE type LE presenting sign features y s (days) Aniridia, nystagmus, 23 years Posterior Posterior 1-1 1 PAX6 Aniridia White British 25 F - glaucoma, foveal N N N Y 4 months subcapsular subcapsular hypoplasia Cleft palate, epilepsy, high Aphakia Aphakia Macular atrophy, myopia, 7 years 9 7 years 8 arched palate, 2-1 2 COL11A1 Stickler syndrome, type II Not Stated 34 F (post- (post- lens subluxation, vitreous N N N months months flattened surgical) surgical) anomaly maxilla, short stature (5'2ft) Anterior segment dysgenesis, pupillary abnormalities including 12 years Posterior Posterior ectopic pupils, ectropion 3-1 3 CPAMD8 Anterior segment dysgenesis 8 Other, Any other 27 F - N N Y N 5 months subcapsular subcapsular UVAE and irodensis, nystagmus, dysplastic optic discs, large corneal diameters Gyrate atrophy of choroid and 23 years 29 years 1 Posterior Posterior Retinal dystrophy, Bipolar 4-1 4 OAT White British 42 F N N N retina 7 months month subcapsular subcapsular exotropia disorder 1 year 6 1 year -

Stereo 3D (Depth) from 2D
Stereo 3D (Depth) from 2D • 3D information is lost by Viewing Stereo projection. Stereograms • How do we recover 3D Autostereograms information? Depth from Stereo Image 3D Model Depth Cues Shadows: Occlusions: Shading: Size Constancy Perspective Illusions (perspective): Height in Plane: Texture Gradient: 3D from 2D + Accommodation • Accommodation (Focus) • Change in lens curvature • Eye Vengeance according to object depth. • Effective depth: 20-300 cm. • Motion. • Stereo Accommodation Eye Vergence • Change in lens curvature • Change in lens curvature according to object depth. according to object depth. • Effective depth: 20-300 cm. • Effective depth: up to 6 m. Motion: Motion: Stereo Vision • In a system with 2 cameras (eyes), 2 different images are captured. • The "disparity" between the images is larger for closer objects: 1 disp ∝ depth • "Fusion" of these 2 images gives disparities depth information. Left Right Right Eye Left Eye Right Eye Left Eye Image Separation for Stereo • Special Glasses • Red/green images with red/green glasses. • Orthogonal Polarization • Alternating Shuttering Optic System Optic System Parlor Stereo Viewer 1850 Viewmaster 1939 ViduTech 2011 Active Shutter System Red/Green Filters Anaglyphs Anaglyphs How they Work Orthogonal Polarization Orthogonal Polarization Linear Polarizers: 2 polarized projectors are used (or alternating polarization) Orthogonal Polarization Orthogonal Polarization Circular Polarizers: Circular Polarizers: Left handed Right handed Orthogonal Polarization TV and Computer Screens Polarized Glasses Circular Polarizer Glasses: Same as polarizers – but reverse light direction Left handed Right handed Glasses Free TV and Glasses Free TV and Computer Screens Computer Screens Parallax Stereogram Parallax Stereogram Parallax Barrier display Uses Vertical Slits Blocks part of screen from each eye Glasses Free TV and Glasses Free TV and Computer Screens Computer Screens Lenticular lens method Lenticular lens method Uses lens arrays to send different Image to each eye. -

Feasibility and Outcome of Descemet Membrane Endothelial Keratoplasty in Complex Anterior Segment and Vitreous Disease
CLINICAL SCIENCE Feasibility and Outcome of Descemet Membrane Endothelial Keratoplasty in Complex Anterior Segment and Vitreous Disease Julia M. Weller, MD, Theofilos Tourtas, MD, and Friedrich E. Kruse, MD escemet membrane endothelial keratoplasty (DMEK), Purpose: Descemet membrane endothelial keratoplasty (DMEK) is Da technique for posterior lamellar keratoplasty, involves becoming the method of choice for treating Fuchs endothelial a graft consisting only of the thin Descemet membrane with dystrophy and pseudophakic bullous keratopathy. We investigated adherent corneal endothelial cells. Introduced in 2006 by whether DMEK can serve as a routine procedure in endothelial Melles et al,1 DMEK is becoming more popular as several decompensation even in complex preoperative situations. studies show its superiority to Descemet stripping automated Methods: Of a total of 1184 DMEK surgeries, 24 consecutive eyes endothelial keratoplasty (DSAEK), regarding visual function 2,3 with endothelial decompensation and complex preoperative situa- and the time of visual rehabilitation after DMEK. However, tions were retrospectively analyzed and divided into 5 groups: group because DMEK grafts are thinner than DSAEK grafts, it is fi 1: irido-corneo-endothelial syndrome (n = 3), group 2: aphakia, more dif cult to handle them and typically takes surgeons subluxated posterior chamber intraocular lens or anterior chamber longer to learn. intraocular lens (n = 6), group 3: DMEK after trabeculectomy (n = In difficult situations, most surgeons prefer DSAEK or 4), group 4: DMEK with simultaneous intravitreal injection (n = 6), penetrating keratoplasty to DMEK because of its possible and group 5: DMEK after vitrectomy (n = 5). Main outcome intraoperative complications. For example, if corneal edema 4 parameters were best-corrected visual acuity, central corneal thick- is advanced, Ham et al recommend performing DSAEK first ness, endothelial cell density, rebubbling rate, and graft failure rate.