Vaccinia (Smallpox) Vaccine
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Poxviruses: Smallpox Vaccine, Its Complications and Chemotherapy
Virus Adaptation and Treatment Dovepress open access to scientific and medical research Open Access Full Text Article R E V IEW Poxviruses: smallpox vaccine, its complications and chemotherapy Mimi Remichkova Abstract: The threat of bioterrorism in the recent years has once again posed to mankind the unresolved problems of contagious diseases, well forgotten in the past. Smallpox (variola) is Department of Pathogenic Bacteria, The Stephan Angeloff Institute among the most dangerous and highly contagious viral infections affecting humans. The last of Microbiology, Bulgarian Academy natural case in Somalia marked the end of a successful World Health Organization campaign of Sciences, Sofia, Bulgaria for smallpox eradication by vaccination on worldwide scale. Smallpox virus still exists today in some laboratories, specially designated for that purpose. The contemporary response in the treatment of the post-vaccine complications, which would occur upon enforcing new programs for mass-scale smallpox immunization, includes application of effective chemotherapeutics and their combinations. The goals are to provide the highest possible level of protection and safety of For personal use only. the population in case of eventual terrorist attack. This review describes the characteristic features of the poxviruses, smallpox vaccination, its adverse reactions, and poxvirus chemotherapy. Keywords: poxvirus, smallpox vaccine, post vaccine complications, inhibitors Characteristics of poxviruses Smallpox (variola) infection is caused by the smallpox virus. This virus belongs to the genus of Orthopoxvirus included in the Poxviridae family. Poxviruses are one of the largest and most complexly structured viruses, known so far. The genome of poxviruses consists of a linear two-chained DNA and its replication takes place in the cytoplasm of the infected cell. -

Vaccinia Belongs to a Family of Viruses That Is Closely Related to the Smallpox Virus
VACCINIA INFECTION What is it? Vaccinia belongs to a family of viruses that is closely related to the smallpox virus. Because of the similarities between the smallpox and vaccinia viruses, the vaccinia virus is used in the smallpox vaccine. When this virus is used as a vaccine, it allows our immune systems to develop immunity against smallpox. The smallpox vaccine does not actually contain smallpox virus and cannot cause smallpox. Vaccination usually prevents smallpox infection for at least ten years. The vaccinia vaccine against smallpox was used to successfully eradicate smallpox from the human population. More recently, this virus has also become of interest due to concerns about smallpox being used as an agent of bioterrorism. How is the virus spread? Vaccinia can be spread by touching the vaccination site before it has fully healed or by touching clothing or bandages that have been contaminated with the live virus during vaccination. In this manner, vaccinia can spread to other parts of the body and to other individuals. It cannot be spread through the air. What are the symptoms of vaccinia? Vaccinia virus symptoms are similar to smallpox, but milder. Vaccinia may cause rash, fever, headache and body aches. In certain individuals, such as those with weak immune systems, the symptoms can be more severe. What are the potential side effects of the vaccinia vaccine for smallpox? Normal reactions are mild and go away without any treatment.These include: Soreness and redness in the arm where the vaccine was given Slightly swollen, sore glands in the armpits Low grade fever One in approximately three people will feel badly enough to miss school, work or recreational activities Trouble sleeping Serious reactions are not very common but can occur in about 1,000 in every 1 million people who are vaccinated for the first time. -
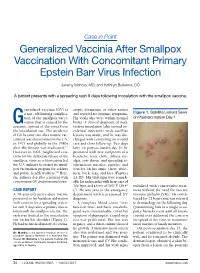
Generalized Vaccinia After Smallpox Vaccination with Concomitant Primary Epstein Barr Virus Infection
Case in Point Generalized Vaccinia After Smallpox Vaccination With Concomitant Primary Epstein Barr Virus Infection Jeremy Mandia, MD; and Kathryn Buikema, DO A patient presents with a spreading rash 9 days following inoculation with the smallpox vaccine. eneralized vaccinia (GV) is atopic dermatitis, or other rashes a rare, self-limiting complica- and reported no systemic symptoms. Figure 1. Satellite Lesions Seen tion of the smallpox vacci- His vitals also were within normal on Postvaccination Day 1 Gnation that is caused by the limits. A clinical diagnosis of inad- systemic spread of the virus from vertent inoculation (also termed ac- the inoculation site. The incidence cidental infection) with satellite of GV became rare after routine vac- lesions was made, and he was dis- cination was discontinued in the U.S. charged with counseling on wound in 1971 and globally in the 1980s care and close follow-up. Two days after the disease was eradicated.1,2 later, on postvaccination day 11, he However in 2002, heightened con- presented with new symptoms of a cerns for the deliberate release of the headache, fever, chills, diffuse my- smallpox virus as a bioweapon led algia, sore throat, and spreading er- the U.S. military to restart its small- ythematous macules, papules, and pox vaccination program for soldiers vesicles on his arms, chest, abdo- and public health workers.3,4 Here, men, back, legs, and face (Figures the authors describe a patient with 2A-2D). His vital signs were remark- concomitant GV and mononucleosis. able for tachycardia with heart rate of 100 bpm and a fever of 103º F (39.4º stabilized with conservative treat- CASE REPORT C). -

Cidofovir Activity Against Poxvirus Infections
Viruses 2010 , 2, 2803-2830; doi:10.3390/v2122803 OPEN ACCESS viruses ISSN 1999-4915 www.mdpi.com/journal/viruses Review Cidofovir Activity against Poxvirus Infections Graciela Andrei * and Robert Snoeck Laboratory of Virology and Chemotherapy, Rega Institute for Medical Research, KULeuven, Minderboredersstraat 10, B-3000 Leuven, Belgium; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +32-16-337372; Fax: +32-16-337340. Received: 10 November 2010; in revised form: 9 December 2010 / Accepted: 10 December 2010 / Published: 22 December 2010 Abstract: Cidofovir [(S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine, HPMPC] is an acyclic nucleoside analog approved since 1996 for clinical use in the treatment of cytomegalovirus (CMV) retinitis in AIDS patients. Cidofovir (CDV) has broad-spectrum activity against DNA viruses, including herpes-, adeno-, polyoma-, papilloma- and poxviruses. Among poxviruses, cidofovir has shown in vitro activity against orthopox [vaccinia, variola (smallpox), cowpox, monkeypox, camelpox, ectromelia], molluscipox [molluscum contagiosum] and parapox [orf] viruses. The anti-poxvirus activity of cidofovir in vivo has been shown in different models of infection when the compound was administered either intraperitoneal, intranasal (aerosolized) or topically. In humans, cidofovir has been successfully used for the treatment of recalcitrant molluscum contagiosum virus and orf virus in immunocompromised patients. CDV remains a reference compound against poxviruses and holds potential for the therapy and short-term prophylaxis of not only orthopox- but also parapox- and molluscipoxvirus infections. Keywords: cidofovir; poxviruses; acyclic nucleoside analog 1. Introduction The antiviral activity of (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine (HPMPC, cidofovir, CDV) (Figure 1) against human cytomegalovirus (HCMV) and other DNA viruses was first Viruses 2010 , 2 2804 reported in 1986 [1]. -

Medical Management of Biological Casualties Handbook
USAMRIID’s MEDICAL MANAGEMENT OF BIOLOGICAL CASUALTIES HANDBOOK Sixth Edition April 2005 U.S. ARMY MEDICAL RESEARCH INSTITUTE OF INFECTIOUS DISEASES FORT DETRICK FREDERICK, MARYLAND Emergency Response Numbers National Response Center: 1-800-424-8802 or (for chem/bio hazards & terrorist events) 1-202-267-2675 National Domestic Preparedness Office: 1-202-324-9025 (for civilian use) Domestic Preparedness Chem/Bio Helpline: 1-410-436-4484 or (Edgewood Ops Center – for military use) DSN 584-4484 USAMRIID’s Emergency Response Line: 1-888-872-7443 CDC'S Emergency Response Line: 1-770-488-7100 Handbook Download Site An Adobe Acrobat Reader (pdf file) version of this handbook can be downloaded from the internet at the following url: http://www.usamriid.army.mil USAMRIID’s MEDICAL MANAGEMENT OF BIOLOGICAL CASUALTIES HANDBOOK Sixth Edition April 2005 Lead Editor Lt Col Jon B. Woods, MC, USAF Contributing Editors CAPT Robert G. Darling, MC, USN LTC Zygmunt F. Dembek, MS, USAR Lt Col Bridget K. Carr, MSC, USAF COL Ted J. Cieslak, MC, USA LCDR James V. Lawler, MC, USN MAJ Anthony C. Littrell, MC, USA LTC Mark G. Kortepeter, MC, USA LTC Nelson W. Rebert, MS, USA LTC Scott A. Stanek, MC, USA COL James W. Martin, MC, USA Comments and suggestions are appreciated and should be addressed to: Operational Medicine Department Attn: MCMR-UIM-O U.S. Army Medical Research Institute of Infectious Diseases (USAMRIID) Fort Detrick, Maryland 21702-5011 PREFACE TO THE SIXTH EDITION The Medical Management of Biological Casualties Handbook, which has become affectionately known as the "Blue Book," has been enormously successful - far beyond our expectations. -

Vaccinia Virus
APPENDIX 2 Vaccinia Virus • Accidental infection following transfer from the vac- cination site to another site (autoinoculation) or to Disease Agent: another person following intimate contact Likelihood of Secondary Transmission: • Vaccinia virus • Significant following direct contact Disease Agent Characteristics: At-Risk Populations: • Family: Poxviridae; Subfamily: Chordopoxvirinae; • Individuals receiving smallpox (vaccinia) vaccination Genus: Orthopoxvirus • Individuals who come in direct contact with vacci- • Virion morphology and size: Enveloped, biconcave nated persons core with two lateral bodies, brick-shaped to pleo- • Those at risk for more severe complications of infec- morphic virions, ~360 ¥ 270 ¥ 250 nm in size tion include the following: • Nucleic acid: Nonsegmented, linear, covalently ᭺ Immune-compromised persons including preg- closed, double-stranded DNA, 18.9-20.0 kb in length nant women • Physicochemical properties: Virus is inactivated at ᭺ Patients with atopy, especially those with eczema 60°C for 8 minutes, but antigen can withstand 100°C; ᭺ Patients with extensive exfoliative skin disease lyophilized virus maintains potency for 18 months at 4-6°C; virus may be stable when dried onto inanimate Vector and Reservoir Involved: surfaces; susceptible to 1% sodium hypochlorite, • No natural host 2% glutaraldehyde, and formaldehyde; disinfection of hands and environmental contamination with soap Blood Phase: and water are effective • Vaccinia DNA was detected by PCR in the blood in 6.5% of 77 military members from 1 to 3 weeks after Disease Name: smallpox (vaccinia) vaccination that resulted in a major skin reaction. • Progressive vaccinia (vaccinia necrosum or vaccinia • In the absence of complications after immunization, gangrenosum) recently published PCR and culture data suggest that • Generalized vaccinia viremia with current vaccines must be rare 3 weeks • Eczema vaccinatum after vaccination. -

(12) Patent Application Publication (10) Pub. No.: US 2012/0009150 A1 WEBER Et Al
US 2012O009 150A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2012/0009150 A1 WEBER et al. (43) Pub. Date: Jan. 12, 2012 (54) DIARYLUREAS FORTREATINGVIRUS Publication Classification INFECTIONS (51) Int. Cl. (76) Inventors: Olaf WEBER, Wulfrath (DE); st 2. CR Bernd Riedl, Wuppertal (DE) ( .01) A63/675 (2006.01) (21) Appl. No.: 13/236,865 A6II 3/522 (2006.01) A6IP 29/00 (2006.01) (22) Filed: Sep. 20, 2011 A6II 3/662 (2006.01) A638/14 (2006.01) Related U.S. Application Data A63L/7056 (2006.01) A6IP3L/2 (2006.01) (63) Continuation of application No. 12/097.350. filed on A6II 3/44 (2006.01) Nov. 3, 2008, filed as application No. PCTAEPO6/ A6II 3/52 (2006.01) 11693 on Dec. 6, 2006. O O (52) U.S. Cl. .......... 424/85.6; 514/350; 514/171; 514/81; (30) Foreign Application Priority Data 514/263.38: 514/263.4: 514/120: 514/4.3: Dec. 15, 2005 (EP) .................................. 05O274513 424/85.7; 514/43 Dec. 15, 2005 (EP). ... O5O27452.1 Dec. 15, 2005 (EP). ... O5O27456.2 Dec. 15, 2005 (EP). ... O5O27458.8 The present invention relates to pharmaceutical compositions Dec. 15, 2005 (EP) O5O27.460.4 for treating virus infections and/or diseases caused by virus Dec. 15, 2005 (EP) O5O27462.O infections comprising at least a diary1 urea compound option Dec. 15, 2005 (EP). ... O5O27465.3 ally combined with at least one additional therapeutic agent. Dec. 15, 2005 (EP). ... O5O274.67.9 Useful combinations include e.g. BAY 43-9006 as a diaryl Dec. -

(12) Patent Application Publication (10) Pub. No.: US 2009/0221523 A1 Tseng Et Al
US 2009.022 1523A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2009/0221523 A1 Tseng et al. (43) Pub. Date: Sep. 3, 2009 (54) NORTH-2'-DEOXY-METHANO- (86). PCT No.: PCT/US2O06/02O894 CARBATHYMIDNES AS ANTIVIRAL AGENTS AGAINST POXVIRUSES S371 (c)(1), (2), (4) Date: Apr. 3, 2009 (76) Inventors: Christopher K. Tseng, Related U.S. Application Data Burtonsville, MD (US); Victor E. (60) Provisional application No. 60/684.811, filed on May Marquez, Montgomery Village, 25, 2005. MD (US) Publication Classification (51) Int. Cl. Correspondence Address: A 6LX 3L/7072 (2006.01) KNOBBE, MARTENS, OLSON & BEAR, LLP A6IP3L/20 (2006.01) 2040 MAINSTREET, FOURTEENTH FLOOR (52) U.S. Cl. .......................................................... S14/SO IRVINE, CA 92.614 (US) (57) ABSTRACT (21) Appl. No.: 11/920,881 A method for the prevention or treatment of poxvirus infec tion by administering an effective amount of an antiviral agent comprising cyclopropanated carbocyclic 2'-deoxy (22) PCT Filed: May 25, 2006 nucleoside to an individual in need thereof is provided. Patent Application Publication Sep. 3, 2009 Sheet 2 of 3 US 2009/022 1523 A1 HSV-2 TK Goalpox TK humonl TK VV TK VOriod TK monkeypox TK fowlpox TK HSV-1 K african swine fever virus TK VZV TK EBV TK FIF2 Patent Application Publication Sep. 3, 2009 Sheet 3 of 3 US 2009/022 1523 A1 CC (N)-MCT CDV VC – -2 48.61 site - 2322 2027 . US 2009/022 1523 A1 Sep. 3, 2009 NORTH-2-DEOXY. breaks depended on the isolation of infected individuals and METHANOCARBATHYMIDNES AS the vaccination of close contacts. -

Here, There, and Everywhere: the Wide Host Range and Geographic Distribution of Zoonotic Orthopoxviruses
viruses Review Here, There, and Everywhere: The Wide Host Range and Geographic Distribution of Zoonotic Orthopoxviruses Natalia Ingrid Oliveira Silva, Jaqueline Silva de Oliveira, Erna Geessien Kroon , Giliane de Souza Trindade and Betânia Paiva Drumond * Laboratório de Vírus, Departamento de Microbiologia, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais: Belo Horizonte, Minas Gerais 31270-901, Brazil; [email protected] (N.I.O.S.); [email protected] (J.S.d.O.); [email protected] (E.G.K.); [email protected] (G.d.S.T.) * Correspondence: [email protected] Abstract: The global emergence of zoonotic viruses, including poxviruses, poses one of the greatest threats to human and animal health. Forty years after the eradication of smallpox, emerging zoonotic orthopoxviruses, such as monkeypox, cowpox, and vaccinia viruses continue to infect humans as well as wild and domestic animals. Currently, the geographical distribution of poxviruses in a broad range of hosts worldwide raises concerns regarding the possibility of outbreaks or viral dissemination to new geographical regions. Here, we review the global host ranges and current epidemiological understanding of zoonotic orthopoxviruses while focusing on orthopoxviruses with epidemic potential, including monkeypox, cowpox, and vaccinia viruses. Keywords: Orthopoxvirus; Poxviridae; zoonosis; Monkeypox virus; Cowpox virus; Vaccinia virus; host range; wild and domestic animals; emergent viruses; outbreak Citation: Silva, N.I.O.; de Oliveira, J.S.; Kroon, E.G.; Trindade, G.d.S.; Drumond, B.P. Here, There, and Everywhere: The Wide Host Range 1. Poxvirus and Emerging Diseases and Geographic Distribution of Zoonotic diseases, defined as diseases or infections that are naturally transmissible Zoonotic Orthopoxviruses. Viruses from vertebrate animals to humans, represent a significant threat to global health [1]. -

Structural and Functional Analysis of the Vaccinia Virus O1 Virulence Protein
Structural and functional analysis of the Vaccinia virus O1 virulence protein by Anastasia C. Weeks June 2017 Director of Thesis: Mark Mannie, Ph.D. Major Department: Biomedical Sciences, Microbiology and Immunology Poxviruses are double-stranded DNA viruses capable of causing disfiguring and deadly disease in a wide range of hosts, from insects to mammals. Orthopoxviruses (OPXV) encode many proteins that are not essential for viral replication, but are responsible for vast differences in pathogenesis. Of the >200 proteins in the prototypical OPXV vaccinia virus (VACV), many remain functionally cryptic. The objective of these studies was to understand how the VACV O1 protein functions by investigating cell-specific effects that may contribute to virulence. The O1L gene is expressed early as the O1 protein, a 78 kDa protein that lacked N-linked glycosylation. These data are the first to demonstrate the reduced ability of an O1 deletion mutant (∆O1) to induce cell migration compared to the parental VACV Western Reserve strain (VACV- WR). ∆O1-infected cell monolayers also exhibited reduced plaque diameter and clearance in plaque foci. These observations indicated that O1 is a significant contributor to VACV cytopathic effects (CPE) in vitro, in agreement with published reports. The results reported herein are the first to describe an altered immunological response with ∆O1, as levels of anti-VACV immunoglobulin significantly increased with ∆O1 infection at a time point (seven days post-infection) when VACV- WR induced VACV-specific antibody levels were comparable to sera from mock-infected mice. ∆O1 was more immunogenic in an ex vivo antigen presentation assay, although mitogen-induced CD4+ T cell activation during ∆O1 infection was equivalent to VACV-WR infection. -

Localized to Vaccination Site (3-12-2003 Version) Low
Legend Tool 1 Morbidity and Mortality Risk Clinical Evaluation Tool for Smallpox Vaccine Adverse Reactions based on clinical presentation. Dermatologic Reactions/ Localized to Vaccination Site (3-12-2003 Version) Low www.bt.cdc.gov/agent/smallpox/vaccination/clineval Moderate NO YES Go to Dermatologic Reactions/ Nontoxic Appearance, Distant from High Close Contact of Vaccine Recipient? Vaccine Recipient? Vaccination Site (or in a Contact) Clinical Evaluation Tool. YES Erythematous Vaccination Normal Vaccination Reaction Severe Vaccination Reaction Reaction of Concern Typical reaction timeline Common signs/symptoms Tape sensitivity Rapid progressive painless extension of Day Description after vaccination Try different tape. Erythema present along lines of central vaccination lesion or progression YES 0 Vaccination - Pruritus Change bandage adhesive tape and no or mild without apparent healing after 15 days. 3-4 Papule - Soreness at frequently, rotate systemic symptoms? Lesion often necrotic. Initially little or no 5-6 Vesicle with surrounding vaccination site bandage, or take a inflammation. May present with few or no erythema - vesicle with - Intense erythema judicious bandage systemic symptoms. depressed center ringing the “holiday” 8-9 Well-formed pustule vaccination site remembering to use NO 12+ Pustule crusts over and - Small papules or other means (e.g. becomes a scab vesicles around long sleeves) to Early Progressive vaccinia (Vaccinia 17-21 Scab detaches revealing scar vaccination lesion prevent contact Erythema with induration, warmth, necrosum, Vaccinia gangrenosum) Timeline may be accelerated (satellite lesions) transmission. Use and pain. May also have regional Go to Clinical Evaluation Tool for in persons with history of prior - Headache antihistamines PRN; lymphadenopathy and fever. Dermatologic Reactions/Toxic smallpox vaccination. -

Abstract Book (PDF)
Abstract Book and Program 7th European Seminar in Virology (EuSeV) “Vaccines and antibodies against viral infections” June 14-16, 2019 Botanical Garden University of Padova 1 7th European Seminar in Virology (EuSeV) “Vaccines and antibodies against viral infections” June 14-16, 2019 Organizers: Gabriella Campadelli-Fiume, University of Bologna Dana Wolf, Hebrew University Jerusalem Michael Kann University of Gothenburg Thomas Mertens, Ulm University Medical Centre Giorgio Palù University of Padova Organizing Secretariat: Arianna Calistri, Ilaria Frasson, Michela Nandi, Marta Trevisan 2 7th. European Seminars in Virology (EuSeV) 2019 Program FRIDAY 14.06.2019 13:45-14:00 Welcome Dana Wolf, Gabriella Campadelli-Fiume, Thomas Mertens, Michael Kann Giorgio Palù Session on Ethical and technological issues about antiviral vaccine and antibodies development Chair: Giorgio Palù 14:00-14:40 Andrea Grignolio, Cognitive bases for vaccine hesitancy Medical Humanities & Bioethics, Vita-Salute San Raffaele University, Milan, Italy [email protected] 14:40-15:20 Rino Rappuoli, Reverse vaccinology 2.0 GSK, Siena, Italy [email protected] 15:20-16:00 Melvin Kohn, MSD’s Investigational Ebola Vaccine Regional Director of Medical Affairs Lead for Vaccines, MSD. [email protected] 16:00-16:30 Discussion 16:30-17:30 Selected oral presentations Francesco Santoro, Human transcriptomic response to vaccination with recombinant VSV expressing Ebola virus Glycoprotein Laboratory of Molecular Microbiology and Biotechnology (LAMMB), Dept. of Medical Biotechnologies,