Distribution of Ascorbate Oxidase in Citrus Fruits
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hooked on Tonics Summer’S Most Iconic Stirred Cocktail, the G&T, Is Ready for a Shake-Up
BOUNTY | GOOD SPIRITS Hooked on Tonics Summer’s most iconic stirred cocktail, the G&T, is ready for a shake-up BY CHRIS HUGHES RECIPES BY EMILY NABORS HALL PHOTOGRAPH BY GREG DUPREE THE MEDITERRANEAN G&T THE ROSE G&T THE HERBAL G&T THE ENGLISHMAN’S G&T Stir together 1/2 cup Mediterranean tonic Muddle 6 Tbsp. (3 oz.) gin and 1 fresh THE FLORAL G&T Combine 6 Tbsp. (3 oz.) gin, 2 cucumber (such as Fever-Tree), 6 Tbsp. (3 oz.) gin, strawberry in a small measuring cup, Stir together 1/2 cup agave tonic water THE BITTERSWEET G&T slices, 3 black peppercorns, and 1 whole 1 Tbsp. (1/2 oz.) white port, and 2 dashes and strain into a tall glass. Top with (such as Q Tonic), 6 Tbsp. (3 oz.) gin, Stir together 1/2 cup elderflower tonic star anise pod in a tall glass, and stir of orange bitters in a tall glass. Firmly 1/2 cup grapefruit tonic water (such as 1 Tbsp. (1/2 oz.) Aperol, 1 Tbsp. (1/2 oz.) water, 6 Tbsp. (3 oz.) gin, 2 Tbsp. (1 oz.) Stir together 1/2 cup Indian tonic water vigorously to infuse flavors, about 30 hit 1 mint sprig against the palm of your Fentimans) and 2 Tbsp. (1 oz.) Lillet Pimm’s, and 2 dashes of lime bitters Suze herbal liqueur, and 1/2 Tbsp. lemon (such as Fever-Tree), 6 Tbsp. (3 oz.) gin, seconds. Fill glass with ice, and top with hand to release oils, and swirl in Blanc. -

Product Codes for Fresh Fruits and Vegetables
S/N HS Code Product Code Product Code Description Unit 1 07019010 HVR0POC POTATO, CHIPPING POTATO TNE 2 07019090 HVR0PO POTATO (OTHER THAN SWEET POTATO) TNE 3 07020000 HVF0TT TOMATO TNE 4 07020000 HVF0TTC TOMATO, CHERRY TOMATO TNE 5 07020000 HVF0TTH TOMATO, HONEY TOMATO TNE 6 07031019 HVB0ON ONION, OTHER THAN SPRING ONION TNE 7 07031019 HVB0ONF ONION FLOWERS TNE 8 07031019 HVB0SC SCALLION TNE 9 07031019 HVB0SO SPRING ONION TNE 10 07031029 HVB0SA SHALLOT TNE 11 07032090 HVB0GL GARLIC TNE 12 07032090 HVB0GLF GARLIC FLOWERS TNE 13 07039090 HVB0CV CHIVE / CHIVE STALK (KOO CHYE/ KOO CHYE HUAY) TNE 14 07039090 HVB0CVY CHIVE YELLOW (JIU HUANG) TNE 15 07039090 HVB0LK LEEK/ LEEK STALK (INCL GARLIC SPROUT) TNE 16 07041010 HVC0CF CAULIFLOWER TNE 17 07041020 HVC0BR BROCCOLI TNE 18 07041020 HVC0CA CALABRESE STICCOLI (CALABRESE TENDERGREEN) TNE 19 07042000 HVC0BP BRUSSEL SPROUT TNE 20 07049010 HVC0CB CABBAGE, ROUND (DRUMHEAD) CABBAGE TNE 21 07049020 HVL0LM CHINESE MUSTARD (BRASSICA JUNCEA) TNE 22 07049020 HVL0WA WASABINA (BRASSICA JUNCEA) TNE 23 07049090 HVC0CBC CABBAGE, LONG CABBAGE (WONGBAK) TNE 24 07049090 HVC0CBR CABBAGE, RED CABBAGE TNE 25 07049090 HVC0KH KOHLRABI TNE 26 07049090 HVL0KL KALE (SPECIFIC TYPES TO BE DECLARED IN PRODUCT TNE DESCRIPTION) 27 07049090 HVL0KLET KALETTES TNE 28 07049090 HVC0BRP SPROUTING BROCCOLI TNE 29 07051100 HVL0LTG LETTUCE GARDEN (CHINESE LETTUCE) TNE 30 07051100 HVL0LTH LETTUCE HEAD (ICEBERG LETTUCE/ENGLISH LETTUCE) TNE 31 07051900 HVL0LTA LETTUCE ASPARAGUS (WO JU) TNE 32 07051900 HVL0LTB LETTUCE BUTTERHEAD TNE 33 -
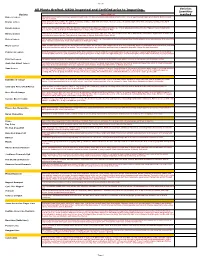
2019 Full Provisional List
Sheet1 All Plants Grafted. USDA inspected and Certified prior to Importing. Varieties Quantities Variety Description required Baboon Lemon A Brazilian lemon with very intense yellow rind and flesh. The flavour is acidic with almost a hint of lime. Tree is vigorous with large green leaves. Both tree and fruit are beautiful. Bearss Lemon 1952. Fruit closely resembles the Lisbon. Very juicy and has a high rind oil content. The leaves are a beautiful purple when first emerging, turning a nice dark green. Fruit is ready from June to December. Eureka Lemon Fruit is very juicy and highly acidic. The Eureka originated in Los Angeles, California and is one of their principal varieties. It is the "typical" lemon found in the grocery stores, nice yellow colour with typical lemon shape. Harvested November to May Harvey Lemon 1948.Having survived the disastrous deep freezes in Florida during the ’60’s and ’70’s. this varieties is known to withstand cold weather. Typical lemon shape and tart, juicy true lemon flavour. Fruit ripens in September to March. Self fertile. Zones 8A-10. Lisbon Lemon Fruit is very juicy and acic. The leaves are dense and tree is very vigorous. This Lisbon is more cold tolerant than the Eureka and is more productive. It is one of the major varieties in California. Fruit is harvested from February to May. Meyer Lemon 1908. Considered ever-bearing, the blooms are very aromatic. It is a lemon and orange hybrid. It is very cold hardy. Fruit is round with a thin rind. Fruit is juicy and has a very nice flavour, with a low acidity. -

0 Golden Hour
GOLDEN HOUR - In the hour after sunrise or the hour before sunset. In light diffused, the party in apricot hues, refused, to end. 0 CONTENTS The Japanese Garden ............................................................................................................ 2 Gin Selection ......................................................................................................................... 8 Gin Serves .......................................................................................................................... 14 Beers .................................................................................................................................... 15 Champagne and Wine Selection .............................................................................. 16 Champagne ........................................................................................................................ 17 Champagne Rosé ............................................................................................................ 19 Vodka ................................................................................................................................... 21 Teqiula ................................................................................................................................. 22 Mezcal .................................................................................................................................. 23 Pisco .................................................................................................................................... -
Holdings of the University of California Citrus Variety Collection 41
Holdings of the University of California Citrus Variety Collection Category Other identifiers CRC VI PI numbera Accession name or descriptionb numberc numberd Sourcee Datef 1. Citron and hybrid 0138-A Indian citron (ops) 539413 India 1912 0138-B Indian citron (ops) 539414 India 1912 0294 Ponderosa “lemon” (probable Citron ´ lemon hybrid) 409 539491 Fawcett’s #127, Florida collection 1914 0648 Orange-citron-hybrid 539238 Mr. Flippen, between Fullerton and Placentia CA 1915 0661 Indian sour citron (ops) (Zamburi) 31981 USDA, Chico Garden 1915 1795 Corsican citron 539415 W.T. Swingle, USDA 1924 2456 Citron or citron hybrid 539416 From CPB 1930 (Came in as Djerok which is Dutch word for “citrus” 2847 Yemen citron 105957 Bureau of Plant Introduction 3055 Bengal citron (ops) (citron hybrid?) 539417 Ed Pollock, NSW, Australia 1954 3174 Unnamed citron 230626 H. Chapot, Rabat, Morocco 1955 3190 Dabbe (ops) 539418 H. Chapot, Rabat, Morocco 1959 3241 Citrus megaloxycarpa (ops) (Bor-tenga) (hybrid) 539446 Fruit Research Station, Burnihat Assam, India 1957 3487 Kulu “lemon” (ops) 539207 A.G. Norman, Botanical Garden, Ann Arbor MI 1963 3518 Citron of Commerce (ops) 539419 John Carpenter, USDCS, Indio CA 1966 3519 Citron of Commerce (ops) 539420 John Carpenter, USDCS, Indio CA 1966 3520 Corsican citron (ops) 539421 John Carpenter, USDCS, Indio CA 1966 3521 Corsican citron (ops) 539422 John Carpenter, USDCS, Indio CA 1966 3522 Diamante citron (ops) 539423 John Carpenter, USDCS, Indio CA 1966 3523 Diamante citron (ops) 539424 John Carpenter, USDCS, Indio -

Asian Citrus Psyllid Control Program in the Continental United States
United States Department of Agriculture Asian Citrus Psyllid Marketing and Regulatory Control Program in the Programs Animal and Continental Plant Health Inspection Service United States and Puerto Rico Environmental Assessment August 2010 Asian Citrus Psyllid Control Program in the Continental United States and Puerto Rico Environmental Assessment August 2010 Agency Contact: Osama El-Lissy Director, Emergency Management Emergency and Domestic Programs Animal Plant Health Inspection Service U.S. Department of Agriculture 4700 River Rd. Unit 134 Riverdale, MD 20737 __________________________________________________________ The U.S. Department of Agriculture (USDA) prohibits discrimination in all its programs and activities on the basis of race, color, national origin, sex, religion, age, disability, political beliefs, sexual orientation, or marital or family status. (Not all prohibited bases apply to all programs.) Persons with disabilities who require alternative means for communication of program information (Braille, large print, audiotape, etc.) should contact USDA’S TARGET Center at (202) 720–2600 (voice and TDD). To file a complaint of discrimination, write USDA, Director, Office of Civil Rights, Room 326–W, Whitten Building, 1400 Independence Avenue, SW, Washington, DC 20250–9410 or call (202) 720–5964 (voice and TDD). USDA is an equal opportunity provider and employer. __________________________________________________________ Mention of companies or commercial products in this report does not imply recommendation or endorsement by the U.S. Department of Agriculture over others not mentioned. USDA neither guarantees nor warrants the standard of any product mentioned. Product names are mentioned solely to report factually on available data and to provide specific information. __________________________________________________________ This publication reports research involving pesticides. All uses of pesticides must be registered by appropriate State and/or Federal agencies before they can be recommended. -

Chemical Variability of Peel and Leaf Essential Oils in the Citrus Subgenus Papeda (Swingle) and Few Relatives
plants Article Chemical Variability of Peel and Leaf Essential Oils in the Citrus Subgenus Papeda (Swingle) and Few Relatives Clémentine Baccati 1, Marc Gibernau 1, Mathieu Paoli 1 , Patrick Ollitrault 2,3 ,Félix Tomi 1,* and François Luro 2 1 Laboratoire Sciences Pour l’Environnement, Equipe Chimie et Biomasse, Université de Corse—CNRS, UMR 6134 SPE, Route des Sanguinaires, 20000 Ajaccio, France; [email protected] (C.B.); [email protected] (M.G.); [email protected] (M.P.) 2 UMR AGAP Institut, Université Montpellier, CIRAD, INRAE, Institut Agro, 20230 San Giuliano, France; [email protected] (P.O.); [email protected] (F.L.) 3 CIRAD, UMR AGAP, 20230 San Giuliano, France * Correspondence: [email protected]; Tel.: +33-495-52-4122 Abstract: The Papeda Citrus subgenus includes several species belonging to two genetically distinct groups, containing mostly little-exploited wild forms of citrus. However, little is known about the potentially large and novel aromatic diversity contained in these wild citruses. In this study, we characterized and compared the essential oils obtained from peels and leaves from representatives of both Papeda groups, and three related hybrids. Using a combination of GC, GC-MS, and 13C-NMR spectrometry, we identified a total of 60 compounds in peel oils (PO), and 76 compounds in leaf oils (LO). Limonene was the major component in almost all citrus PO, except for C. micrantha and C. hystrix, where β-pinene dominated (around 35%). LO composition was more variable, with different Citation: Baccati, C.; Gibernau, M.; major compounds among almost all samples, except for two citrus pairs: C. -

CITRUS BUDWOOD Annual Report 2017-2018
CITRUS BUDWOOD Annual Report 2017-2018 Citrus Nurseries affected by Hurricane Irma, September 2017 Florida Department of Agriculture and Consumer Services Our Vision The Bureau of Citrus Budwood Registration will be diligent in providing high yielding, pathogen tested, quality budlines that will positively impact the productivity and prosperity of our citrus industry. Our Mission The Bureau of Citrus Budwood Registration administers a program to assist growers and nurserymen in producing citrus nursery trees that are believed to be horticulturally true to varietal type, productive, and free from certain recognizable bud-transmissible diseases detrimental to fruit production and tree longevity. Annual Report 2018 July 1, 2017 – June 30, 2018 Bureau of Citrus Budwood Registration Ben Rosson, Chief This is the 64th year of the Citrus Budwood Registration Program which began in Florida in 1953. Citrus budwood registration and certification programs are vital to having a healthy commercial citrus industry. Clean stock emerging from certification programs is the best way to avoid costly disease catastrophes in young plantings and their spread to older groves. Certification programs also restrict or prevent pathogens from quickly spreading within growing areas. Regulatory endeavors have better prospects of containing or eradicating new disease outbreaks if certification programs are in place to control germplasm movement. Budwood registration has the added benefit in allowing true-to-type budlines to be propagated. The selection of high quality cultivars for clonal propagation gives growers uniform plantings of high quality trees. The original mother stock selected for inclusion in the Florida budwood program is horticulturally evaluated for superior performance, either by researchers, growers or bureau staff. -

List of Citrus Fruits
Common Taxonomic SNo Notes name(s) name/constituents Yellowish-orange in colour, about the size of grapefruit and oblate in shape. 1 Amanatsu Citrus natsudaidai The fruit contains 12 segments and about 30 seeds. Balady citron 2 Palestinian Citrus medica Grown in Israel and used for Jewish ritual purposes. citron Bergamot 3 Citrus bergamia orange Bitter orange Seville orange Sour orange 4 Bigarade Citrus × aurantium orange Marmalade orange 5 Blood orange Citrus × sinensis Buddha's hand Citrus medica var. 6 Bushukan sarcodactylis Fingered citron Calamondin × Citrofortunella 7 Calamansi mitis Citrus reticulata × 8 Cam sành maxima 9 Citron Citrus medica Citrus subg. Papeda indicates the subgenus Papeda of the genus Citrus, with citrus species native to Asia.The papeda group includes some of the most Citrus subg. tropical, and also some of the most frost-tolerant citrus plants. They are 10 Papeda cultivated far less often than other citrus, though they will all hybridize with other citrus. This group contains about 15 species. 11 Clementine Citrus reticulata Corsican 12 citron Found in lowland subtropical rainforest and dry rainforest areas of Queensland and New South Wales, Australia. Early settlers consumed the 13 Desert Lime Citrus glauca fruit and retained the trees when clearing for agriculture. Commercial uses include boutique marmalade and restaurant dishes, and is exported for such. 14 Etrog Citrus medica The finger lime has been recently popularised as a gourmet bushfood. 15 Finger lime Citrus australasica Finger lime is thought to -

EU Project Number 613678
EU project number 613678 Strategies to develop effective, innovative and practical approaches to protect major European fruit crops from pests and pathogens Work package 1. Pathways of introduction of fruit pests and pathogens Deliverable 1.3. PART 7 - REPORT on Oranges and Mandarins – Fruit pathway and Alert List Partners involved: EPPO (Grousset F, Petter F, Suffert M) and JKI (Steffen K, Wilstermann A, Schrader G). This document should be cited as ‘Grousset F, Wistermann A, Steffen K, Petter F, Schrader G, Suffert M (2016) DROPSA Deliverable 1.3 Report for Oranges and Mandarins – Fruit pathway and Alert List’. An Excel file containing supporting information is available at https://upload.eppo.int/download/112o3f5b0c014 DROPSA is funded by the European Union’s Seventh Framework Programme for research, technological development and demonstration (grant agreement no. 613678). www.dropsaproject.eu [email protected] DROPSA DELIVERABLE REPORT on ORANGES AND MANDARINS – Fruit pathway and Alert List 1. Introduction ............................................................................................................................................... 2 1.1 Background on oranges and mandarins ..................................................................................................... 2 1.2 Data on production and trade of orange and mandarin fruit ........................................................................ 5 1.3 Characteristics of the pathway ‘orange and mandarin fruit’ ....................................................................... -

Just Fruits "Out of Stock" Update As of January 1St, 2019 (Fruit Trees, Berry Plants, & Nut Trees Only
Just Fruits "Out of Stock" Update As of January 1st, 2019 (Fruit Trees, Berry Plants, & Nut Trees Only. Ornamentals & Herbs will be added on future lists) We gladly take recommendations of new varieties that you are searching for or would enjoy if we carried. We also enjoy information on growers that may grow or have available varieties that we currently do not have in stock. Please submit them through our "Contact Us" page at www.justfruitsandexotics.com OR reach out to us by messaging us on Facebook at Facebook.com/justfruit Category Item Price* Status (Month/Year)* Removed Variety. Currently don't plan on growing anymore. Natchez is a similar variety that customers have Berry Plants / Blackberry Bushes Chickasaw Blackberry Bush 1 GAL, 1-2 FT $14.99 found better. Removed Variety. Currently don't plan on growing anymore. Natchez is a similar variety that customers have Berry Plants / Blackberry Bushes Chickasaw Blackberry Bush 3 GAL, 2-3 FT $24.99 found better. Berry Plants / Blackberry Bushes Kiowa Blackberry Bush 3 GAL, 2-3 FT $24.99 Currently Available in Other Sizes Berry Plants / Blackberry Bushes Natchez Blackberry Bush 3 GAL, 2-3 FT $24.99 Currently Available in Other Sizes Berry Plants / Blackberry Bushes Osage Blackberry Bush 3 GAL, 2-3 FT $24.99 Currently Available in Other Sizes Berry Plants / Blackberry Bushes Ouachita Blackberry Bush 3 GAL, 2-3 FT $24.99 Currently Available in Other Sizes Berry Plants / Blackberry Bushes Prime-Ark 45 Blackberry Bush 3 GAL, 2-3 FT $24.99 Currently Available in Other Sizes Berry Plants / Blackberry -

Table of Japanese Cuisine
Table of Japanese Cuisine Wakayama Prefecture 1 Catalogue of Wakayama Prefecture’s Specialties の食卓 Table of Japanese Cuisine Grand mountains, the sea and the powerful Kuroshio Current, bright sunlight, and wisdom of people living in such a wonderful environment – Wakayama Prefecture is home to a wide variety of specialties developed through the rich nature, culture, and people. 2 Climate: Blessings from the Earth and the Sea 4 Premium Wakayama 10 Wakayama’s distinctive specialties are recommended with a focus The gourmet food of Kishu comes from its bountiful nature. on safety and reliability. Food Culture: Food History Traced to “Kinokuni” 6 Kishu Ume-Plum Burger 12 A pairing of Wakayama ume-plums and hamburgers, developed Japanese cuisine originated from the enterprising spirit of people in Kishu. with overseas sales in mind. Taste of the Hometown: Honor and Pride of the Hometown 8 Wakayama Punch 13 As a kingdom of fruit, Wakayama is proud to provide this While people in the past demonstrated their wisdom, people today pursue outstanding product filled with love for our hometowns. even deeper, which underpins Japanese cuisine. < Products Catalogue > Fruit 14 Vegetables 19 Livestock Products 22 Seafood 24 Processed foods 29 Wakayama Seasonal Product Calendar 36 Share of Wakayama’s Farm and Marine Production 38 Monde Selection 39 ※ Produce amounts and other information in this brochure are based on statistics in 2016. 3 Specialties from Wakayama lend flair to the Climate “Table of Japanese Cuisine.” W AKAYAMA In Wakayama, while the climate is moderate in some parts, the deep mountains The gourmet food of generate changes in temperature in other areas.