Pharmacological Research
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

List of Union Reference Dates A
Active substance name (INN) EU DLP BfArM / BAH DLP yearly PSUR 6-month-PSUR yearly PSUR bis DLP (List of Union PSUR Submission Reference Dates and Frequency (List of Union Frequency of Reference Dates and submission of Periodic Frequency of submission of Safety Update Reports, Periodic Safety Update 30 Nov. 2012) Reports, 30 Nov. -

In February 2013, Glaxosmithkline (GSK) Announced a Commitment To
In February 2013, GlaxoSmithKline (GSK) announced a commitment to further clinical transparency through the public disclosure of GSK Clinical Study Reports (CSRs) on the GSK Clinical Study Register. The following guiding principles have been applied to the disclosure: Information will be excluded in order to protect the privacy of patients and all named persons associated with the study Patient data listings will be completely removed* to protect patient privacy. Anonymized data from each patient may be made available subject to an approved research proposal. For further information please see the Patient Level Data section of the GSK Clincal Study Register. Aggregate data will be included; with any direct reference to individual patients excluded *Complete removal of patient data listings may mean that page numbers are no longer consecutively numbered CONFIDENTIAL GM2004/00056/00GM2004/00056/00 SAM40040SAM40040 The GlaxoSmithKline group of companies A twenty-four week, randomised, double-dummy, double-blind, parallel group study to compare the occurrence of exacerbations between SERETIDE DISKUS 50/250µg 1 inhalation bd and formoterol/budesonide Breath-Actuated Dry Powder Inhaler 4.5/160µg 2 inhalations bd in subjects with moderate to severe asthma. Clinical Study Report for Study SAM40040 (Development Phase IV) Document Number: GM2004/00056/00 Compound Number: CCI18781/ GR33343 Investigational Product: SERETIDE Generic Drug Name: salmeterol/fluticasone propionate combination Indication Studied: Asthma Initiation Date: 26 Nov 2001 Completion Date: 13 Jan 2003 Early Termination Date: N/A Date of Report: August 2004 Sponsor Signatory: (and Medical Officer) Vice President European Clinical Respiratory Medicines Development Centre This study was performed in compliance with Good Clinical Practices including the archiving of essential documents. -

Moderní Antihistaminika V Léčbě Alergie – Současné Trendy V Symptomatické Terapii Alergických Onemocnění
100 Hlavní téma Moderní antihistaminika v léčbě alergie – současné trendy v symptomatické terapii alergických onemocnění Jaroslava Braunová, Mojmír Račanský Oddělení alergologie a klinické imunologie FNOL, Ústav imunologie UP Olomouc Alergická onemocnění jsou problémem současné medicíny. Nejde o pouhé projevy sezónní rýmy, s nimiž se do jisté míry setkal snad každý, ale také o závažné stavy, jako je anafylakticky šok a exacerbace bronchiálního astmatu. Nezanedbatelnou kapitolu tvoří problematika různých kožních projevů přecitlivělosti. Vždy však působí postiženému značné nepříjemnosti. Spolu s rostoucí incidencí těchto alergolo- gických diagnóz pochopitelně stoupá i poptávka po jejich účinné léčbě. Za kauzální léčbu považujeme alergenovou imunoterapii (AIT). Ta však není indikována pro všechny pacienty a všechny alergologické diagnózy. Vyžaduje vysokou compliance ze strany nemocného, přesné určení spouštěče alergické reakce u daného pacienta a existenci dostupného standardizovaného alergenu. Symptomatická terapie antihistaminiky představuje jednu ze zásadních možností ovlivnění rozvoje alergické reakce přímo na periferním histaminovém receptoru H1. Klíčová slova: alergie, symptomatická terapie, antihistaminika, alergický zánět. Modern antihistamines in treating allergies: current trends in symptomatic therapy of allergic conditions Allergic diseases are a serious problem in modern medicine. It is not only a question of pollinosis, but also of other allergic diseases such as bronchial asthma, atopic dermatitis, urticaria, angioedema or -

Obecná Alergologie, Alergie - Pojem
12.02.2017 Obecná alergologie, Alergie - pojem (alergie, „nealergie“, alergeny, léčba neadekvátní reakce imunitního systému typu zánětu alergií a alergické stavy) na jinak neškodnou cizorodou látku (alergen), má s přihlédnutím k dětskému věku objektivní i subjektivní složku: - objektivně jsou známky poškození některých orgánů (sliznice nosu, spojivky, kůže, bronchy, ..) - subjektivně jsou nepříjemně vnímané poruchy funkce takto postižených orgánů Alergik = pacient s klinickými příznaky reakce přecitlivosti navozené imunolog.mechanismy. MUDr.Jiří NEVRLKA Poliklinika Zahradníkova 2/8 BRNO ambulance pro alergologii a klinickou imunologii Alergie pozitivita v testech na alergeny !! Alergie - pojem Alergie - pojem imunopatologické reakce „nepravé alergie a pseudoalergie“ časný typ Reakce I.typu - mediovaná protilátkami IgE, • IgE non dependentní alergie (pozdní typ) - vyjádřena u osob s atopickou reaktivitou Alergolog - reakce nezprostředkovaná IgE, ale Ag-specif.T-lymfocyty - nejčastější typ alergie (alergie v užším smyslu) navazující na IgE mechanismus .. např. potraviny, alergie na lepek - inhalační alergie, alergie hmyzí jed, anafylaxe, (atopická dermatitida).. dominantní mechanismus .. např. kontaktní alergeny, léky, celiakie Reakce II.typu - mediovaná non IgE protilátkami - cytotoxické protilátky, např. transfuzní reakce x blokující protilátky, např. • Zkřížená reaktivita: myastenia gravis x stimulační protilátky, např. Graves-Basedowova choroba - reakce na antigeny strukturálně podobné reálnému alergenu Reakce III.typu – mediovaná -

Azelastine Hydrochloride
568 Antihistamines been withdrawn from the market in most countries because of the Preparations treatment of non-allergic rhinitis in adults and children risk of adverse effects. USP 31: Azatadine Maleate Tablets. aged 12 years and over. The dose is 2 sprays into each Astemizole has been given in an oral dose of 10 mg once daily, Proprietary Preparations (details are given in Part 3) nostril twice daily. In the treatment of conjunctivitis, or 5 mg daily in children aged 6 to 12 years. These doses must Austral.: Zadine; Canad.: Optimine; Hong Kong: Zadine†; Malaysia: azelastine is licensed in the UK for the treatment of not be exceeded because of the risk of cardiac arrhythmias with Zadine†; Mex.: Idulamine†; NZ: Zadine†; Singapore: Zadine†; Spain: seasonal allergic conjunctivitis in adults and children higher doses. Lergocil. aged 4 years and over and for the treatment of perenni- The active metabolite of astemizole, tecastemizole (norastemi- Multi-ingredient: Braz.: Cedrin; Canad.: Trinalin; Mex.: Trinalin†; al allergic conjunctivitis in adults and children aged 12 zole) has been investigated for the treatment of allergic rhinitis. Spain: Atiramin; Idulanex; USA: Rynatan†; Trinalin†. years and over. In the USA, it is licensed for the treat- Preparations ment of allergic conjunctivitis in adults and children USP 31: Astemizole Tablets. aged 3 years and over. Regardless of the age and indi- Azelastine Hydrochloride cation, a 0.05% solution is instilled into each eye twice Proprietary Preparations (details are given in Part 3) (BANM, USAN, rINNM) daily; this may be increased to four times daily in se- Arg.: Alermizol†; Astezol†; Cezane†; Mudantil†; Cz.: Hismanal†; Gr.: Mibiron†; Tulipe-R†; Tyrenol†; Waruzol†; India: Astizole; Stemiz†; Mex.: A-5610 (azelastine or azelastine hydrochloride); Atselastiinihy- vere conditions. -

Prezentace Aplikace Powerpoint
Immunostimulation and Immunomodulation - Vaccination, Biological Treatment, Current Possibilities of Immunological Intervention Mgr. Michal Křupka, Ph.D. Pharmacotherapy in immunology Allergy therapy - antihistamines, cromoglycans, corticoids, local alpha and betamimetics, biological therapy, specific immunotherapy Immunosuppression - NSAIDs, corticosteroids, calcineurin inhibitors, mTOR inhibitors, cyclophosphamide, azathioprine, mycophenolate, methotrexate Immunostimulation non-specific - levamizole, isoprinosine specific - vaccination substitution - immunoglobulins Therapy of allergic diseases Antihistamines used to treat allergic diseases 1. First generation Antihistaminics Highly sedative: Diphenhydramine, Dimenhydrinate, Promethazine, Hydroxyzine Moderately sedative: Pheniramine, Cyproheptadine, Meclizine, Buclizine, Cinnarazine Mild sedative: Chlorpheniramine, Dexchlorpehniramine, Dimethindene, Triprolidine… Only injectable antihistaminic in the Czech republik – Bisulepin (Dithiaden). 2. Second generation Antihistaminics Fexofenadine, Loratadine, Desloratadine, Cetrizine, Levocetrizine, Azelastine, Mizolastine, Rupatadine…. Sodium cromoglycate Derivative of Khellin, substance conained in corrot like plant Khella (Ammi visnaga), for centuries used as myorelaxans (Egypt). Stabilizes membrane of basophiles - prevents degranulation and release of mediators of allergic reaction. Acts „a step earlier“ than antihistamines. Application in nasal sprays, nebulizers, drops but also orally. Commercial preparations - local - Allergocrom, Cromohexal, -
Poison Or Antibiotic? a Guide to "Class" Entries
Poison or Antibiotic? A Guide to “Class” Entries Preface Most entries in the Poisons List, i.e. the Schedule 10, and the Schedules 1, 2 and 3 to the Pharmacy and Poisons Regulations (Cap. 138A) are in the form of individual drugs and their salts, e.g. “Abacavir; its salts”. However, some entries are in the form of a “class”, e.g. “Barbituric acid; its salts; its derivatives …”. In such cases, it is not always clear which drugs are members of the class (e.g. amobarbital, barbital, pentobarbital, phenobarbital, etc. are poisons, being derivatives of barbituric acid). Likewise, the Antibiotics Ordinance (Cap. 137) applies to the substances specified in Schedule 1 to the Antibiotics Regulations, to their salts and derivatives, and to the salts of such derivatives. Again, it is not always clear which drugs are derivatives of an antibiotic named in the Schedule (e.g. demeclocycline, doxycycline, tigecycline, etc. are antibiotics, being derivatives of “Tetracycline” named in the Schedule). This Guide provides a list of such drugs which are available as registered pharmaceutical products in Hong Kong. Drugs which are not available as registered pharmaceutical products in Hong Kong are also included in this Guide as far as possible. It should be noted that it is not possible to compile a complete list of all these drugs, simply because there is no limit to the number of “derivatives” a parent chemical can have. This Guide should be read in conjunction with the Schedules 1, 2, 3, and 10 to the Pharmacy and Poisons Regulations, and Schedule 1 to the Antibiotics Regulations, if the poison/antibiotic classification of a particular pharmaceutical product is to be determined. -
(Microsoft Powerpoint
Antihistamines Histamine • is released from mast cells granules by exocytosis (activation of phospholipase C a ↑ Ca 2+ ) Stimuli : imunological: antigen + IgE physical , chemical or mechanical cell damage drugs Histamin receptors • 4 subtypes (H 1 –H4) • G protein-coupled receptors • their stimulation results in increase in cellular concentration of Ca 2+ ions H1 receptors • postsynaptic , Gq-protein ↑ phospholipase C → ↑ IP3 and DAG → ↑ Ca 2+ Lo cation : endothel , smooth muscles (vessels , bronchi , uterus, GIT), peripheral neuron ending, CNS (!!!) Effect : smooth muscle contraction (bronchi, uterus, ileum) vasodilatation of minor vessels (↓BP, reddening of skin) increase in vessel permeability ( swelling ) irritation of peripheral neuron endings (itching, even pain) excitation of CNS H2 receptors • postsynaptic , Gs-protein ↑ activity of adenylate cyclase → ↑cAMP Location: stomach mucosa , heart , vessels , immune system Effect : in stomach: gastric acid, pepsine, intrinsic factor secretion slower and longer vasodilatation + inotropic , + chronotropic effect H3 receptors 2+ • presynaptic , Gi protein → inhibition of N -type Ca channels → ↓ cellular Ca 2+ • feedback inhibition of histamine release • heterorec eptors , ↓ release of other neurotransmitters Location: mainly in CNS (but in PNS tissues as well ) Effect : sedation negative chronotropic effect bronchoconstriction H4 receptors • possibly isoform of H3 Location : • eosinophiles, basophiles, bone marrow, thymus, intestine , spleen Effect : – influencing activity of immune system -

Modern Antihistamines in Treating Allergies: Current Trends in Symptomatic Therapy of Allergic Conditions Allergic Diseases Are a Serious Problem in Modern Medicine
AKTUÁLNÍ FARMAKOTERAPIE MODERNÍ ANTIHISTAMINIKa v lÉčBě aLERGIe – SOUčASNÉ TRENDy v sYMPTOMATICKÉ TERAPII ALERGICKÝCH ONEMOCNěNÍ Moderní antihistaminika v léčbě alergie – současné trendy v symptomatické terapii alergických onemocnění Jaroslava Braunová, Mojmír Račanský Oddělení alergologie a klinické imunologie FNOL, Ústav imunologie UP Olomouc Alergická onemocnění jsou problémem současné medicíny. Nejde o pouhé projevy sezónní rýmy, s nimiž se do jisté míry setkal snad každý, ale také o závažné stavy, jako je anafylakticky šok a exacerbace bronchiálního astmatu. Nezanedbatelnou kapitolu tvoří problematika různých kožních projevů přecitlivělosti. Vždy však působí postiženému značné nepříjemnosti. Spolu s rostoucí incidencí těchto alergologických diagnóz pochopitelně stoupá i poptávka po jejich účinné léčbě. Za kauzální léčbu považujeme alergenovou imunoterapii (AIT). Ta však není indiko- vána pro všechny pacienty a všechny alergologické diagnózy. Vyžaduje vysokou compliance ze strany nemocného, přesné určení spouštěče alergické reakce u daného pacienta a existenci dostupného standardizovaného alergenu. Symptomatická terapie antihistaminiky představuje jednu ze zásadních možností ovlivnění rozvoje alergické reakce přímo na periferním histaminovém receptoru H1. Klíčová slova: alergie, symptomatická terapie, antihistaminika, alergický zánět. Modern antihistamines in treating allergies: current trends in symptomatic therapy of allergic conditions Allergic diseases are a serious problem in modern medicine. It is not only a question of pollinosis, -

Moderní Antihistaminika V Léčbě Alergie – Současné Trendy V Symptomatické Terapii Alergických Onemocnění
PŘEHLEDOVÉ ČLÁNKY MODERNÍ ANTIHISTAMINIKA V LÉčBě ALERGIE – SOUčASNÉ TRENDY V SYMPTOMATICKÉ TERAPII ALERGICKÝCH ONEMOCněNÍ Moderní antihistaminika v léčbě alergie – současné trendy v symptomatické terapii alergických onemocnění MUDr. Jaroslava Braunová, Ph.D., MUDr. Mojmír Račanský Oddělení alergologie a klinické imunologie fakultní nemocnice Olomouc, Ústav imunologie Univerzity Palackého Olomouc Alergická onemocnění jsou problémem současné medicíny. Nejde o pouhé projevy sezónní rýmy, s nimiž se do jisté míry setkal snad kaž- dý, ale také o závažné stavy, jako je anafylaktický šok a exacerbace bronchiálního astmatu. Nezanedbatelnou kapitolu tvoří problematika různých kožních projevů přecitlivělosti. Vždy však působí postiženému značné nepříjemnosti. Spolu s rostoucí incidencí těchto alergolo- gických diagnóz pochopitelně stoupá i poptávka po jejich účinné léčbě. Za kauzální léčbu považujeme alergenovou imunoterapii (AIT). Ta však není indikována pro všechny pacienty a všechny alergologické diagnózy. Vyžaduje vysokou compliance ze strany nemocného, přesné určení spouštěče alergické reakce u daného pacienta a existenci dostupného standardizovaného alergenu. Symptomatická terapie antihistaminiky představuje jednu ze zásadních možností ovlivnění rozvoje alergické reakce přímo na periferním histaminovém receptoru H1. Klíčová slova: alergie, symptomatická terapie, antihistaminika, alergický zánět. Modern antihistamines in treating allergies: current trends in symptomatic therapy of allergic conditions Allergic diseases are a challenge for contemporary medicine. They involve not only the mere manifestations of seasonal rhinitis, encountered to a certain degree by nearly everyone, but also serious conditions, such as anaphylactic shock and bronchial asthma exacerbation. A non-negligible aspect is the issue of various skin manifestations of hypersensitivity. They are invariably associat- ed with a great deal of discomfort for the sufferer. With a rising incidence of these allergic conditions, the demand for effective treatment naturally rises. -

Současné Trendy V Léčbě a Diagnostice Chronické Nespavosti Souborný Článek
Čes a slov Psychiat 2020; 116(3): 139–149 SOUčASNÉ TRENDY V LéčBě a diaGNOSTICE CHRONICKÉ NEspaVOSTI souborný článek David Kec1,2 Ondřej Ludka3,4 SOUHRN SUMMARY Veronika Hamerníková5 Jan Kubánek6 Kec D, Ludka O, Hamerníková V, Ku- Kec D, Ludka O, Hamerníková V, 1,2 Josef Bednařík bánek J, Bednařík J, Vlčková E. Součas- Kubánek J, Bednařík J, Vlčková E. Cur- Eva Vlčková1,2 né trendy v léčbě a diagnostice chro- rent trends in the treatment and diag- nické nespavosti nosis of chronic insomnia 1Lékařská fakulta MU v Brně 2Neurologická klinika FN Brno Přehledné sdělení shrnuje aktuální mož- The review summarizes the current 3I. interní kardio-angiologická nosti diagnostiky a léčby chronické ne- possibilities of diagnosis and treat- klinika FN u sv. Anny v Brně spavosti a reflektuje specifika českého ment of chronic insomnia and reflects 4Mezinárodní centrum klinického prostředí (dostupnost jednotlivých léků, the specifics of the Czech environment výzkumu FN u sv. Anny v Brně možnosti kognitivně-behaviorální tera- (the availability of individual drugs, 5Odborný psycholog, pie). Vychází z recentně aktualizovaných possibilities of cognitive-behavioral Opletalova 600/6, Brno mezinárodních klinických doporučení therapy). It is based on recently updat- 6Psychiatr v Priessnitzových v oblasti somnologie i doporučení od- ed international clinical recommenda- léčebných lázních, Jeseník borných společností České lékařské spo- tions in the field of somnology (Euro- lečnosti Jana Evangelisty Purkyně. pean Sleep Research Society, American Kontaktní adresa: Doporučené postupy Evropské spo- Academy of Sleep Medicine) as well prof. MUDr. Ondřej Ludka, Ph.D. lečnosti pro výzkum spánku (European as on the recommendations of profes- I. interní kardio-angiologická klinika Sleep Research Society), Americké aka- sional societies of the Czech Medical FN u sv. -
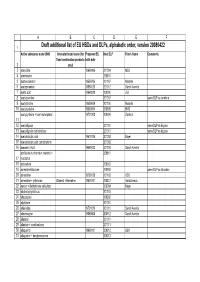
Old Substances Alphabetic Order 20080422
ABCDEF 1 Draft additional list of EU HBDs and DLPs, alphabetic order, version 20080422 Active substance name (INN) Innovator brand name (for Proposed EU Next DLP Firm's Name Comments fixed combination products birth date 2 only) 3 aceclidine 19660406 201004 MSD 4 acemetacin 200810 5 acenocoumarol 19550706 201107 Novartis 6 acetazolamide 19551026 201012 Sanofi Aventis 7 acetic acid 19640928 200909 J&J 8 acetylcarnitine 201012 same DLP as carnitine 9 acetylcholine 19650609 201106 Novartis 10 acetylcysteine 19630914 200909 BMS acetylcysteine + tuaminohelptane 19701203 200909 Zambon 11 12 acetyldigoxin 201010 same DLP as digoxin 13 acetyldigoxin combinations 201010 same DLP as digoxin 14 acetylsalicylic acid 19570105 201002 Bayer 15 acetylsalicylic acid combinations 201002 16 acexamic Acid 19680412 201104 Sanofi Aventis acriflavinium chloride + menthol + 200811 17 resorcinol 18 acrivastine 200912 19 adrenalin+lidocaine 200903 same DLP as lidocaine 20 adrenaline 19780125 201103 UCB 21 adrenaline + prilocaine Citanest -Adrenaline 19630101 200812 AstraZeneca 22 aescin + diethylamine salicylate 200904 Bayer 23 alcohol polyvinilicus 201101 24 alfacalcidol 200902 25 algeldrate 201001 26 alibendole 19701019 201010 Sanofi Aventis 27 alimemazine 19560605 200912 Sanofi Aventis 28 allantoin 201011 29 allantoin + combinations 201011 30 allopurinol 19660101 200912 GSK 31 allopurinol + benzbromarone 200912 ABCDEF Active substance name (INN) Innovator brand name (for Proposed EU Next DLP Firm's Name Comments fixed combination products birth date 2 only)