Multidimensional Analysis of 16 Glucose Isomers by Ion Mobility Spectrometry M
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Electronic Supplementary Information
Electronic Supplementary Material (ESI) for Chemical Science. This journal is © The Royal Society of Chemistry 2019 Electronic Supplementary Information Poly(ionic liquid)s as a Distinct Receptor Material to Create Highly- Integrated Sensing Platform for Efficiently Identifying a Myriad of Saccharides Wanlin Zhang, Yao Li, Yun Liang, Ning Gao, Chengcheng Liu, Shiqiang Wang, Xianpeng Yin, and Guangtao Li* *Corresponding authors: Guangtao Li ([email protected]) S1 Contents 1. Experimental Section (Page S4-S6) Materials and Characterization (Page S4) Experimental Details (Page S4-S6) 2. Figures and Tables (Page S7-S40) Fig. S1 SEM image of silica colloidal crystal spheres and PIL inverse opal spheres. (Page S7) Fig. S2 Adsorption isotherm of PIL inverse opal. (Page S7) Fig. S3 Dynamic mechanical analysis and thermal gravimetric analysis of PIL materials. (Page S7) Fig. S4 Chemical structures of 23 saccharides. (Page S8) Fig. S5 The counteranion exchange of PIL photonic spheres from Br- to DCA. (Page S9) Fig. S6 Reflection and emission spectra of spheres for saccharides. (Page S9) Table S1 The jack-knifed classification on single-sphere array for 23 saccharides. (Page S10) Fig. S7 Lower detection concentration at 10 mM of the single-sphere array. (Page S11) Fig. S8 Lower detection concentration at 1 mM of the single-sphere array. (Page S12) Fig. S9 PIL sphere exhibiting great pH robustness within the biological pH range. (Page S12) Fig. S10 Exploring the tolerance of PIL spheres to different conditions. (Page S13) Fig. S11 Exploring the reusability of PIL spheres. (Page S14) Fig. S12 Responses of spheres to sugar alcohols. (Page S15) Fig. -

Hydrocolloids Structure and Properties the Building Blocks for Structure Timothy J
Hydrocolloids Structure and Properties The building blocks for structure Timothy J. Foster 18 month Meeting, Unilever Vlaardingen, March 29‐31, 2010 Manufactured Materials Foams Emulsions Natural Materials This shows a layer of onion (Allium) cells. Targeting Hydrocolloids For Specific Applications: Approach Material Ingredient Properties Microstructure Oral Process Response Packaging Distribution Storage Process Controlled oral response Process (mouth/gut) Controlling Structure (taste, flavour, texture) CONSTRUCTION DECONSTRUCTION Designed texture/ Ingredient In body functionality Ingredient appearance/ (enzymes) behaviour Interaction with body mucins Reconstruction (associative and new phase separation) Microstructure changes as a Impact on / of starting function of enzyme action materials / structures Re-assembly of structures as a function of digestion breakdown products and body secretions (micelle formation, delivery vehicles) Single Biopolymer systems Hydrocolloid Structure/ Function Need: - define biopolymer primary structure - understand the nature of the interaction / rates - understand the solvent effects - measure material properties - test influence of primary structure variation and changes in environmental conditions on mechanical properties. Hydrocolloid Materials & Function Gelling Thickening Emulsification Pectin Pectin • Gelatin Alginate Alginate • Milk proteins Starch Starch • Egg proteins Agar LBG Carrageenan • Soya proteins Guar gum Gellan • Pea proteins Gelatin Xanthan • Gum Arabic Milk proteins Egg proteins Hydrocolloid -

1) Which of the Following Biomolecules Simply Refers to As “Staff of Life”? (A) Lipids (B) Proteins (C) Vitamins (D) Carbohydrates Sol: (D) Carbohydrates
1) Which of the following Biomolecules simply refers to as “Staff of life”? (a) Lipids (b) Proteins (c) Vitamins (d) Carbohydrates Sol: (d) Carbohydrates. 2) Which of the following is the simplest form of carbohydrates? (a) Carboxyl groups (b) Aldehyde and Ketone groups (c) Alcohol and Carboxyl groups (d) Hydroxyl groups and Hydrogen groups Sol: (b) Aldehyde and Ketone groups. 3) Which of the following monosaccharides is the majority found in the human body? (a) D-type (b) L-type (c) LD-types (d) None of the above Sol: (a) D-type. 4) Which of the following is the most abundant biomolecule on the earth? (a) Lipids (b) Proteins (c) Carbohydrates (d) Nucleic acids. Sol: (c) Carbohydrates. 5) Which of the following are the major functions of Carbohydrates? (a) Storage (b) Structural framework (c) Transport Materials (d) Both Storage and structural framework Sol: (d) Both Storage and structural framework. 6) Which of the following is the general formula of Carbohydrates? (a) (C4H2O)n (b) (C6H2O)n (c) (CH2O)n (d) (C2H2O)n COOH Sol: (c) (CH2O)n. 7) Which of the following is the smallest carbohydrate – triose? (a) Ribose (b) Glucose (c) Glyceraldehyde (d) Dihydroxyacetone Sol: (c) Glyceraldehyde. 8) Which of the following is a reducing sugar? (a) Dihydroxyacetone (b) Erythrulose (c) Glucose (d) All of the above Sol: (c) Glucose. 9) Which of the following is an example of Epimers? (a) Glucose and Ribose (b) Glucose and Galactose (c) Galactose, Mannose and Glucose (d) Glucose, Ribose and Mannose Sol: (b) Glucose and Galactose 10) Which of the following has reducing properties? (a) Mucic acid (b) Glucaric acid (c) Gluconic acid (d) Glucuronic acid Sol: (d) Glucuronic acid. -

WO 2013/070444 Al 16 May 2013 (16.05.2013) W P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2013/070444 Al 16 May 2013 (16.05.2013) W P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A23G 4/00 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (21) International Application Number: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, PCT/US20 12/062043 DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, 26 October 2012 (26.10.2012) KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, (25) Filing Language: English NO, NZ, OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, (26) Publication Language: English RW, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, (30) Priority Data: ZM, ZW. 61/556,546 7 November 20 11 (07. 11.201 1) US (84) Designated States (unless otherwise indicated, for every (71) Applicant (for all designated States except US): WVI. kind of regional protection available): ARIPO (BW, GH, WRIGLEY JR. COMPANY [US/US]; 1132 Blackhawk GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, SZ, TZ, Street, Chicago, IL 60642 (US). -

Monosaccharides: a Tof-SIMS Reference Accession #: 01592, 01593, 01594, 01595, 01596, 01597, 01598, Spectra Database
Monosaccharides: A ToF-SIMS reference Accession #: 01592, 01593, 01594, 01595, 01596, 01597, 01598, spectra database. II. Positive polarity 01599, 01600, 01601, 01602, a) 01603, 01604, 01605, 01606, Laetitia Bernard, Rowena Crockett, and Maciej Kawecki 01607, 01608, 01609, 01610 Laboratory of Nanoscale Materials Science, Empa, CH-8600 Dübendorf, Switzerland Technique: SIMS (Received 20 August 2019; accepted 30 October 2019; published 3 December 2019) Host Material: Silicon (100) wafer The number of time-of-flight secondary ion mass spectrometry studies on biological tissues and Instrument: IONTOF TOF-SIMS.5 cells has strongly increased since the development of primary ion sources that allow not only ele- Major Species in Spectra: C, H, O, (N) mental but also molecular analysis. Substantial fragmentation during ionic bombardment results in a Minor Species in Spectra: Na, K large number of peaks, rendering data analysis complex. Complete and trustable sets of reference spectra for the main biological building blocks, i.e., amino acids, monosaccharides, fatty acids, and Published Spectra: 19 nucleotides, are required. This work aims to provide an accurate and extensive library of reference Spectra in Electronic Record: 19 + spectra for monosaccharides, measured with the Bi3 primary ion. Here (Paper II), the positive polar- Published Figures: 20 ity spectra and lists of associated characteristic fragments are presented. Published by the AVS. Spectral Category: Reference https://doi.org/10.1116/1.5125103 Keywords: ToF-SIMS, carbohydrate, sugar, monosaccharide, mass spectrometry, fragmentation INTRODUCTION Fluka and all others from Sigma Aldrich. Each powder was dissolved in freshly de-ionized H2O (resistivity >18.2 MΩ cm) Monosaccharides are the building blocks of structural polymers at a concentration of 0.1M. -

Food Carbohydrates: Monosaccharides and Oligosaccharides
Paper No. 01 Paper Title: Food Chemistry Module-04: Food carbohydrates: Monosaccharides and Oligosaccharides Monosaccharides The simplest form of carbohydrates is the monosaccharide. Monosaccharides are either aldoses or ketoses. Aldoses such as glucose consists of a carbon backbone and a carbonyl group (C=O) located at the end of the chain. Ketoses such as fructose consists of a carbon backbone with a carbonyl group located at any other carbon in the chain. The remaining carbon atoms are bound to hydroxyl groups (-OH). Monosaccharide classifications based on the number of carbons Number Category of Examples Name Carbons 4 Tetrose Erythrose, Threose 5 Pentose Arabinose, Ribose, Ribulose, Xylose, Xylulose, Lyxose Allose, Altrose, Fructose, Galactose, Glucose, Gulose, Idose, 6 Hexose Mannose, Sorbose, Talose, Tagatose 7 Heptose Sedoheptulose, Mannoheptulose Monosaccharides Three common sugars glucose, galactose and fructose share the same molecular formula: C6H12O6. Because of their six carbon atoms, each is a hexose. Although all three share the same molecular formula, the arrangement of atoms differs in each case. Substances such as these three, which have identical molecular formulas but different structural formulas, are known as structural isomers. Glucose "Blood sugar" is the immediate source of energy for cellular respiration. Glucose, which is also referred to as dextrose, is a moderately sweet sugar found in vegetables and fruit. When glucose is fermented by the enzyme zymase, in yeast, it results in the formation of carbon dioxide and ethyl alcohol. It is the basic structure to which all carbohydrates are reduced to in the end, for transport via the bloodstream and use by the cells of the body. -
![25 05.Html.Ppt [Read-Only]](https://docslib.b-cdn.net/cover/0806/25-05-html-ppt-read-only-1790806.webp)
25 05.Html.Ppt [Read-Only]
25.5 A Mnemonic for Carbohydrate Configurations The Eight D-Aldohexoses CH O H OH CH2OH The Eight D-Aldohexoses All CH O Altruists Gladly Make Gum In H OH Gallon CH2OH Tanks The Eight D-Aldohexoses All Allose CH O Altruists Altrose Gladly Glucose Make Mannose Gum Gulose In Idose H OH Gallon Galactose CH2OH Tanks Talose The Eight D-Aldohexoses Allose CH O Altrose Glucose Mannose Gulose Idose H OH Galactose CH2OH Talose The Eight D-Aldohexoses Allose CH O Altrose Glucose Mannose Gulose H OH Idose H OH Galactose CH2OH Talose The Eight D-Aldohexoses Allose CH O Altrose Glucose Mannose Gulose HO H Idose H OH Galactose CH2OH Talose The Eight D-Aldohexoses Allose CH O Altrose Glucose Mannose Gulose H OH Idose H OH Galactose CH2OH Talose The Eight D-Aldohexoses Allose CH O Altrose Glucose Mannose H OH Gulose H OH Idose H OH Galactose CH2OH Talose The Eight D-Aldohexoses Allose CH O Altrose Glucose Mannose HO H Gulose H OH Idose H OH Galactose CH2OH Talose The Eight D-Aldohexoses Allose CH O Altrose Glucose Mannose Gulose HO H Idose H OH Galactose CH2OH Talose The Eight D-Aldohexoses Allose CH O Altrose Glucose Mannose H OH Gulose HO H Idose H OH Galactose CH2OH Talose The Eight D-Aldohexoses Allose CH O Altrose Glucose Mannose HO H Gulose HO H Idose H OH Galactose CH2OH Talose The Eight D-Aldohexoses Allose CH O Altrose Glucose Mannose H OH Gulose H OH Idose H OH Galactose CH2OH Talose The Eight D-Aldohexoses Allose CH O Altrose Glucose H OH Mannose H OH Gulose H OH Idose H OH Galactose CH2OH Talose The Eight D-Aldohexoses Allose CH O Altrose -

20H-Carbohydrates.Pdf
Carbohydrates Carbohydrates are compounds that have the general formula CnH2nOn Because CnH2nOn can also be written Cn(H2O)n, they appear to be “hydrates of carbon” Carbohydrates are also called “sugars” or “saccharides” Carbohydrates can be either aldoses (ald is for aldehyde and ose means a carbohydrate) or ketoses (ket is for ketone) OH OH O OH CH2OH CH2OH OHC HOH2C OH OH OH OH An Aldose A Ketose (D-Glucose) (D-Fructose) Carbohydrates Due to the multiple chiral centers along a linear carbon chain for carbohydrates, Emil Fischer developed the “Fischer Projection” in order to represent these compounds Remember how to draw a Fischer projection: 1) View the linear carbon chain along the vertical axis (always place the more oxidized carbon [aldehyde in an aldose] towards the top) 2) The horizontal lines are coming out of the page toward the viewer 3) Will need to change the viewpoint for each carbon so the horizontal substituents are always pointing towards the viewer CHO OH OH H OH HO H CH2OH = OHC H OH OH OH H OH CH2OH Emil Fischer (1852-1919) Carbohydrates The aldoses are thus all related by having an aldehyde group at one end, a primary alcohol group at the other end, and the two ends connected by a series of H-C-OH groups CHO CHO CHO CHO CHO H OH H OH H OH H OH HO H CH2OH H OH H OH H OH HO H CH2OH H OH H OH HO H CH2OH H OH HO H CH2OH CH2OH Aldotriose Aldotetrose Aldopentose Aldohexose Aldohexose D-glyceraldehyde D-erythose D-ribose D-allose L-allose The D-aldoses are named according to glyceraldehyde, the D refers to the configurational -

United States Patent (19 11) 4,312,979 Takemoto Et Al
United States Patent (19 11) 4,312,979 Takemoto et al. 45 Jan. 26, 1982 54 POLYSACCHARIDES CONTAINING 58) Field of Search ......................... 536/1, 18, 114, 4; ALLOSE 435/101 (75) Inventors: Hisao Takemoto; Tatsuo Igarashi, (56) References Cited both of Shin-Nanyo, Japan U.S. PATENT DOCUMENTS 73 Assignee: Toyo Soda Manufacturing Co., Ltd., 3,711,462 l/1973 Abdo et al. ............................. 536/1 Tokyo, Japan 4,186,025 l/1980 Kang et al. ............................. 536/1 Primary Examiner-Johnnie R. Brown (21) Appl. No.: 30,444 Attorney, Agent, or Firm-Scully, Scott, Murphy & Presser 22 Filed: Apr. 16, 1979 57 ABSTRACT 30 Foreign Application Priority Data A new polysaccharide including allose as a constituent Apr. 20, 1978 JP Japan .................................. 53.45918 sugar and further characterized by galactose as a major Dec. 5, 1978 JP Japan ................................ 53-149715 constituent sugar is described. The polysaccharide is produced extracellularly by cultivation of Pseudomonas 51) Int. Cl. ............................................... CO7H1/08 viscogena strains in nutrient medium. 52 U.S. C. ...... 0 a a 4 536/1; 435/72; 435/101; 536/114 6 Claims, 2 Drawing Figures U.S. Patent Jan. 26, 1982 Sheet 1 of 2 4,312,979 8 O O Sn Cd O ar - O - 3 CD won L Cd O CO ve O Cd Cd cN O O O N O O o O o O O. d o o O a. 3 c) do N. ud to at Y on 9 (%) AONWLLIWSNW U.S. Patent Jan. 26, 1982 Sheet 2 of 2 4,312,979 3 s 8 un co, t OO O) O S s S 8 & O O O O O r ONWSOS9W 4,312,979 1. -

Biochemistry Introductory Lecture Dr
Biochemistry Introductory lecture Dr. Munaf S. Daoud Carbohydrates (CHO) Definition: Aldehyde or Ketone derivatives of the higher polyhydric alcohols or compounds which yield these derivatives on hydrolysis. Classification: (mono, di, oligo, poly) saccharide. Monosaccharides: Can be classified as trioses, tetroses, pentoses, hexoses and heptoses depending upon the number of carbon atoms, and as aldoses or ketoses, depending upon whether they have an aldehyde or ketone group. Aldehyde (-CHO) Aldoses Ketone (-C=O) Ketoses Polysaccharides (glycans): Homopolysaccharides (homoglycans): e.g. starch, glycogen, inulin, cellulose, dextrins, dextrans. Heteropolysaccharides (heteroglycans): e.g. mucopolysaccharides (MPS) or glycosaminoglycans. Function of CHO: 1) Chief source of energy (immediate and stored energy). 2) Constituent of compound lipids and conjugated protein. 3) Structural element like cellulose. 4) Drugs like cardiac glycosides and antibodies. 5) Lactating mammary gland (Lactose in milk). 6) Synthesis of other substances like fatty acids, cholesterol, amino acids…etc. by their degradation products. 7) Constituent of mucopolysaccharides. 1 1) Stereo-isomerism Stereo-isomers: D-form, L-form 2) Optical isomers (optical activity) Enantiomers: dextrorotatory (d or + sign) Levorotatory (l or – sign) Racemic (d l) 3) Cyclic structures or open chain 4) Anomers and Anomeric carbon OH on carbon number 1, if below the plane then its -form, if above the plane then -form. Mutarotation: the changes of the initial optical rotation that takes place -
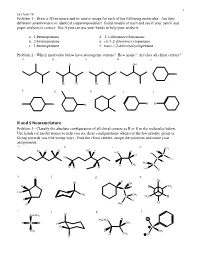
R and S Nomemclature Problem 3 - Classify the Absolute Configuration of All Chiral Centers As R Or S in the Molecules Below
1 Lecture 16 Problem 1 - Draw a 3D structure and its mirror image for each of the following molecules. Are they different (enantiomers) or identical (superimposable)? Build models of each and see if your pencil and paper analysis is correct. See if you can use your hands to help your analysis. a. 1-bromopentane d. 1,1-dibromocyclopentane b. 2-bromopentane e. cis-1,2-dibromocyclopentane c. 3-bromopentane f. trans-1,2-dibromocyclopentane Problem 2 - Which molecules below have stereogenic centers? How many? Are they all chiral centers? a. b. c. d. e. Cl OH Br Br Cl Br Br f. g. h. i. j. Br Br Br Br R and S Nomemclature Problem 3 - Classify the absolute configuration of all chiral centers as R or S in the molecules below. Use hands (or model atoms) to help you see these configurations whenever the low priority group is facing towards you (the wrong way). Find the chiral centers, assign the priorities and make your assignments. a. b. c. d. CH3 Cl H H H3C H H H H3C Br Cl C HO C CH3 I H H3C H CH3 C H H H H3C H e. O f. g. h. H Br Br OH H H H CH3 C H3C CH3 CH3 C H3C H H H H C H Cl 3 i. j. k. l. Cl CH2CH3 Br H3CH2C H C Br H CH3 Cl S CH3 D CH3 H CH3 O H 2 Lecture 16 Pi Bond Priority Problem 4 - Evaluate the order of priority in each part. -

Pyranose Ring Conformation: 1 Pyranose Ring Conformation: 4 #06
#06. 2012-01-20 Quiz 1: Thursday 26 Jan 2012 from 11 AM to 12 noon GG Building Ground Floor #06. 2012-01-20 Last class... Clarification on torsion potential: periodicity 2 and 3 Conformer selection, active/inactive conformations, activation by ligands, etc. - alternative models Inter- and intra-molecular interactions Non-covalent / non-bonded interactions Bonded and non-bonded atoms Hard-sphere approximation Steric effect Preference of trans over gauche conformation for bulky groups #06. 2012-01-20 Monosubstituted cyclohexanes Me Me Bulky group is axially Bulky group is equatorially oriented: gauche to both oriented: trans to both vicinal carbon atoms vicinal carbon atoms #06. 2012-01-20 Monosubstituted cyclohexanes Me Me Bulky group is axially Bulky group is equatorially oriented: gauche to both oriented: trans to both vicinal carbon atoms vicinal carbon atoms #06. 2012-01-20 Cis 1,2-disubstituted cyclohexane Me 4 5 6 Me 3 2 1 #06. 2012-01-20 Trans 1,4-disubstituted cyclohexane 4 5 6 Me Me 3 2 1 #06. 2012-01-20 What governs the conformational preferences? Conformation “a” Conformation “b” Unfolded Folded (protein, DNA, RNA) Monomers (homo/hetero) Oligomer(s) (protein, lipid) A + B A·B (binding) ∆∆∆G = ∆∆∆H – T ∆∆∆S Steric criterion – (approximation of) van der Waals interactions Often, van der Waals contribution is not predominant #06. 2012-01-20 Conformation of 1,3,5-trineopentylbenzene CH 2-tBu tBu-H2C CH 2-tBu Two neopentyl groups are on one side, All the three neopentyl groups are on third on the other side of the ring the same side of the ring view along the plane of the ring Nishio & Hirota (1989) Tetrahedron 45:7201 #06.