Uniqueness of the Gut Microbiome of the Galápagos Vampire Finch Alice J
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ecuador & the Galapagos Islands
Ecuador & the Galapagos Islands - including Sacha Lodge Extension Naturetrek Tour Report 29 January – 20 February 2018 Medium Ground-finch Blue-footed Booby Wire-tailed Manakin Galapagos Penguin Green Sea Turtle Report kindly compiled by Tour participants Sally Wearing, Rowena Tye, Debbie Hardie and Sue Swift Images courtesy of David Griffiths, Sue Swift, Debbie Hardie, Jenny Tynan, Rowena Tye, Nick Blake and Sally Wearing Naturetrek Mingledown Barn Wolf’s Lane Chawton Alton Hampshire GU34 3HJ UK T: +44 (0)1962 733051 E: [email protected] W: www.naturetrek.co.uk Tour Report Ecuador & the Galapagos Islands - including Sacha Lodge Extension Tour Leader in the Galapagos: Juan Tapia with 13 Naturetrek Clients This report has kindly been compiled by tour participants Sally Wearing, Rowena Tye, Debbie Hardie and Sue Swift. Day 1 Monday 29th January UK to Quito People arrived in Quito via Amsterdam with KLM or via Madrid with Iberia, while Tony came separately from the USA. Everyone was met at the airport and taken to the Hotel Vieja Cuba; those who were awake enough went out to eat before a good night’s rest. Day 2 Tuesday 30th January Quito. Weather: Hot and mostly sunny. The early risers saw the first few birds of the trip outside the hotel: Rufous- collared Sparrow, Great Thrush and Eared Doves. After breakfast, an excellent guide took us on a bus and walking tour of Quito’s old town. This started with the Basilica del Voto Nacional, where everyone marvelled at the “grotesques” of native Ecuadorian animals such as frigatebirds, iguanas and tortoises. -

Darwin's Theory of Evolution Through Natural Selection
THE EVOLUTION OF LIFE NOTEBOOK Darwin’s Theory of Evolution Through Natural Selection OBSERVING PHENOMENA Phenomenon: Darwin found many kinds of finches with different sized and shaped beaks on the different islands of the Galápagos. 1. What questions do you have about this phenomenon? 1 - Darwin’s Voyage on the Beagle 1. What did Darwin see in South America that surprised him? 2. What was Lyell’s argument about Earth’s land features and what did it cause Darwin to question about the mountains? © Teachers’ Curriculum Institute Darwin’s Theory of Evolution Through Natural Selection 1 NOTEBOOK 2 - Darwin Visits the Galápagos Islands 1. What did many the species of animals in the Galapagos island resemble? 2. What is a trait? Give one example of a trait. 2 Darwin’s Theory of Evolution Through Natural Selection © Teachers’ Curriculum Institute NOTEBOOK INVESTIGATION 1 1. Which tool is a model for which bird’s beak? Match them below. Tools A. toothpick B. tweezers C. nail clippers D. pliers Tool Finch Large ground finch Vegetarian finch Cactus finch Woodpecker finch 2. Keep track of how many of each bird there are after each round. Station 1 Finch Before Round 1 After Round 1 After Round 2 Large ground finch 1 Vegetarian finch 1 Cactus finch 1 Woodpecker finch 1 Station 2 Finch Before Round 1 After Round 1 After Round 2 Large ground finch 1 Vegetarian finch 1 Cactus finch 1 Woodpecker finch 1 © Teachers’ Curriculum Institute Darwin’s Theory of Evolution Through Natural Selection 3 NOTEBOOK Station 3 Finch Before Round 1 After Round 1 After Round 2 Large ground finch 1 Vegetarian finch 1 Cactus finch 1 Woodpecker finch 1 Station 4 Finch Before Round 1 After Round 1 After Round 2 Large ground finch 1 Vegetarian finch 1 Cactus finch 1 Woodpecker finch 1 3. -

26Sor Em2rray /Mmucd
D4006- Gut Microbiota Analysis UC Davis MMPC - Microbiome & Host Response Core Contents 1 Methods: 1 1.1 Sequencing . .1 1.2 Data processing . .1 2 Summary of Findings: 2 2.1 Sequencing analysis . .2 2.2 Microbial diversity . .2 2.2.1 Alpha Diversity . .2 2.2.2 Beta Diversity . 10 2.3 Data analysis using taxa abundance data . 13 2.3.1 Stacked bar graphs of Taxa abundances at each level . 14 A Appendix 1 (Taxa Abundance Tables) 30 B Appendix 2 (Taxa removed from filtered datasets at each level) 37 References: 43 Core Contacts: Helen E. Raybould, Ph.D., Core Leader ([email protected]) Trina A. Knotts, Ph.D., Core Co-Leader ([email protected]) Michael L. Goodson, Ph.D., Core Scientist ([email protected]) Client(s): Kent Lloyd, DVM PhD ;MMRRC; UC Davis Project #: MBP-2079 MMRRC strain ID: MMRRC_043514 Animal Information: The strain was donated to the MMRRC by Russell Ray at Baylor College of Medicine. Fecal samples were obtained from animals housed under the care of Russell Ray at Baylor College of Medicine. 1 Methods: Brief Project Description: MMRRC strains are often contributed to the MMRRC to fulfill the resource sharing aspects of NIH grants. Since transporting mice to another facilty often causes a microbiota shift, having a record of the original fecal microbiota from the donor institution where the original phenotyping or testing was performed may prove helpful if a phenotype is lost after transfer. Several MMRRC mouse lines were selected for fecal microbiota profiling of the microbiota. Table 1: Animal-Strain Information X.SampleID TreatmentGroup Animal_ID Genotype Line Sex MMRRC.043514.Mx054 MMRRC.043514_Het_M Mx054 Het MMRRC.043514 M MMRRC.043514.Mx415 MMRRC.043514_Het_M Mx415 Het MMRRC.043514 M 1 1.1 Sequencing Frozen fecal or regional gut samples were shipped on dry ice to UC Davis MMPC and Host Microbe Systems Biology Core. -

Can Darwin's Finches and Their Native Ectoparasites Survive the Control of Th
Insect Conservation and Diversity (2017) 10, 193–199 doi: 10.1111/icad.12219 FORUM & POLICY Coextinction dilemma in the Galapagos Islands: Can Darwin’s finches and their native ectoparasites survive the control of the introduced fly Philornis downsi? 1 2 MARIANA BULGARELLA and RICARDO L. PALMA 1School of Biological Sciences, Victoria University of Wellington, Wellington, New Zealand and 2Museum of New Zealand Te Papa Tongarewa, Wellington, New Zealand Abstract. 1. The survival of parasites is threatened directly by environmental alter- ation and indirectly by all the threats acting upon their hosts, facing coextinction. 2. The fate of Darwin’s finches and their native ectoparasites in the Galapagos Islands is uncertain because of an introduced avian parasitic fly, Philornis downsi, which could potentially drive them to extinction. 3. We documented all known native ectoparasites of Darwin’s finches. Thir- teen species have been found: nine feather mites, three feather lice and one nest mite. No ticks or fleas have been recorded from them yet. 4. Management options being considered to control P. downsi include the use of the insecticide permethrin in bird nests which would not only kill the invasive fly larvae but the birds’ native ectoparasites too. 5. Parasites should be targeted for conservation in a manner equal to that of their hosts. We recommend steps to consider if permethrin-treated cotton sta- tions are to be deployed in the Galapagos archipelago to manage P. downsi. Key words. Chewing lice, coextinction, Darwin’s finches, dilemma, ectoparasites, feather mites, Galapagos Islands, permethrin, Philornis downsi. Introduction species have closely associated species which are also endangered (Dunn et al., 2009). -
Fe(II) Fe(III)
Southern Illinois University Carbondale OpenSIUC Publications Department of Microbiology 2006 Microbes Pumping Iron: Anaerobic Microbial Iron Oxidation and Reduction Karrie A. Weber University of California - Berkeley Laurie A. Achenbach Southern Illinois University Carbondale, [email protected] John D. Coates University of California - Berkeley Follow this and additional works at: https://opensiuc.lib.siu.edu/micro_pubs Published in Nature Reviews Microbiology 4, 752-764 (October 2006), http://dx.doi.org/10.1038/ nrmicro1490. This Article is brought to you for free and open access by the Department of Microbiology at OpenSIUC. It has been accepted for inclusion in Publications by an authorized administrator of OpenSIUC. For more information, please contact [email protected]. 1 Microbes Pumping Iron: 2 Anaerobic Microbial Iron Oxidation and Reduction 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 Karrie A. Weber1, Laurie A. Achenbach2, and John D. Coates1* 18 19 20 21 22 23 1Department of Plant and Microbial Biology 24 271 Koshland Hall 25 University of California, Berkeley 26 Berkeley, CA 94720 27 28 29 2Department of Microbiology 30 Southern Illinois University 31 Carbondale, IL 62901 32 33 34 35 Corresponding Author 36 Tel: (510) 643-8455 37 Fax: (510) 642-4995 38 e-mail: [email protected] 39 1 1 Abstract 2 Iron (Fe) has long been a recognized physiological requirement for life, yet its role for a 3 broad diversity of microorganisms persisting in water, soils, and sediments extends well-beyond 4 a nutritional necessity. Microorganisms from within both the domain Archaea and Bacteria are 5 capable of metabolically exploiting the favorable redox potential between the Fe(III)/Fe(II) 6 couple. -

Supplementary Material Impacts of Radiation on the Bacterial And
Supplementary Material Impacts of radiation on the bacterial and fungal microbiome of small mammals in the Chernobyl Exclusion Zone Rachael E. Antwis, Nicholas A. Beresford, Joseph A. Jackson, Ross Fawkes, Catherine L. Barnett, Elaine Potter, Lee Walker, Sergey Gaschak, Michael D. Wood Table S1 Sample sizes of host species used in the study, and associated sex, total absorbed dose rate, burn and site categories for these samples. Animals estimated to receive total absorbed dose rates of <4 µGy h-1 were assigned ‘low’, those with estimated dose rates of 4-42 µGy h-1 assigned ‘medium’, and those >42 µGy h-1 assigned to the ‘high’ category. All samples from 2017 were collected within the Red Forest but from areas that had experienced different degrees of damage from forest fires. Samples from 2018 were either collected within or outside the Red Forest. Sampling information Sex Total absorbed dose category Burn category Site category Year Sample Host species Total n Female Male Unidentified Low Medium High Burnt Burnt Unburnt Inside Red Outside type (regrowth) (minimal Forest Red regrowth) Forest 2017 Faeces Bank vole 22 5 16 1 0 0 22 3 1 18 All - 2017 Faeces Striped field mouse 29 10 18 1 0 7 22 15 0 14 All - 2017 Faeces Wood mouse 27 6 20 1 0 0 27 2 25 0 All - 2017 Faeces Yellow-necked mouse 58 28 29 1 0 7 51 19 17 22 All - 2018 Gut Bank vole 142 59 83 0 42 64 36 - - - 34 108 (caecum) RESULTS Differences in community composition between host species and sample types Wood mice and yellow-necked mice had a similar bacterial community composition to one another (Figure 1a), with the exception of the family Helicobacteraceae (Z = 2.982, p = 0.023; Figure 1c). -

Darwins Finches and Their Diet Niches: the Sympatric Coexistence Of
doi: 10.1111/jeb.12383 Darwin’s finches and their diet niches: the sympatric coexistence of imperfect generalists L. F. DE LEON*†,J.PODOS‡,T.GARDEZI†,A.HERREL§ &A.P.HENDRY† *Centro de Biodiversidad y Descubrimiento de Drogas, Instituto de Investigaciones Cientıficas y Servicios de Alta Tecnologıa (INDICASAT-AIP), Panama 5, Panama †Redpath Museum & Department of Biology, McGill University, Montreal, QC, Canada ‡Department of Biology, University of Massachusetts, Amherst, MA, USA §UMR 7179 C.N.R.S/M.N.H.N., Departement d’Ecologie et de Gestion de la Biodiversite, 57 rue Cuvier, Case postale 55, 75231, Paris Cedex 5, France Keywords: Abstract adaptive radiation; Adaptive radiation can be strongly influenced by interspecific competition ecological speciation; for resources, which can lead to diverse outcomes ranging from competitive Galapagos; exclusion to character displacement. In each case, sympatric species are generalist; expected to evolve into distinct ecological niches, such as different food Geospiza; types, yet this expectation is not always met when such species are exam- ground finches; ined in nature. The most common hypotheses to account for the coexistence niche partitioning; of species with substantial diet overlap rest on temporal variation in niches resource use; (often diets). Yet spatial variation in niche overlap might also be important, specialist; pointing to the need for spatiotemporal analyses of diet and diet overlap sympatry. between closely related species persisting in sympatry. We here perform such an analysis by characterizing the diets of, and diet overlap among, four sympatric Darwin’s ground finch species at three sites and over 5 years on a single Galapagos island (Santa Cruz). -

Adobe PDF, Job 6
Noms français des oiseaux du Monde par la Commission internationale des noms français des oiseaux (CINFO) composée de Pierre DEVILLERS, Henri OUELLET, Édouard BENITO-ESPINAL, Roseline BEUDELS, Roger CRUON, Normand DAVID, Christian ÉRARD, Michel GOSSELIN, Gilles SEUTIN Éd. MultiMondes Inc., Sainte-Foy, Québec & Éd. Chabaud, Bayonne, France, 1993, 1re éd. ISBN 2-87749035-1 & avec le concours de Stéphane POPINET pour les noms anglais, d'après Distribution and Taxonomy of Birds of the World par C. G. SIBLEY & B. L. MONROE Yale University Press, New Haven and London, 1990 ISBN 2-87749035-1 Source : http://perso.club-internet.fr/alfosse/cinfo.htm Nouvelle adresse : http://listoiseauxmonde.multimania. -
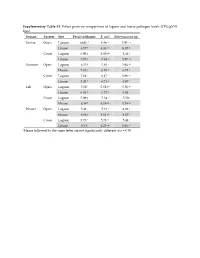
Supplementary Table S1. Fisher Pairwise Comparisons of Lagoon and House Pathogen Levels (CFU/Gvss Log10) Season System Site Fecal Coliforms E
Supplementary Table S1. Fisher pairwise comparisons of lagoon and house pathogen levels (CFU/gVSS log10) Season System Site Fecal coliforms E. coli Enterococcus sp. Spring Open Lagoon 6.00 g1 5.46 hi 5.91 cd House 6.57 e 6.33 cd 6.35 b Cover Lagoon 6.00 g 5.60 gh 5.44 f House 7.73 a 7.63 a 5.87 cd Summer Open Lagoon 6.35 f 5.83 f 5.94 cd House 7.83 a 6.10 e 6.73 a Cover Lagoon 7.04 c 6.47 c 5.86 cd House 7.42 b 6.71 b 6.07 c Fall Open Lagoon 5.58 i 5.58 gh 5.56 ef House 6.81 d 6.72 b 6.01 c Cover Lagoon 5.99 g 5.34 i 5.50 f House 6.38 f 6.19 de 5.74 de Winter Open Lagoon 5.41 j 5.13 j 4.88 g House 6.06 g 5.61 gh 6.67 a Cover Lagoon 5.73 h 5.73 fg 5.44 f House 6.34 f 6.25 de 5.86 cd 1Means followed by the same letter are not significantly different at p = 0.05 Supplementary Table S2. Relative abundances of OTUs identified using universal bacterial primer set, presented as relative abundances (%) Only bacterial families are counted in Figure 5 and discussion involving bacterial family identification. # Identified in # Identified from all 8 All 16 All #OTU Table SpOLRA SuOLRA FOLRA WOLRA SpCLRA SuCLRA FCLRA WCLRA SpOHRA SuOHRA FOHRA WOHRA SpCHRA SuCHRA FCHRA WCHRA all samples lagoon samples Samples Lagoons Notes Key Patulibacteraceae 0 9.93739E‐05* 0.000134 0.000116 0 0 0 0 0 0 0.000452 0 0 0 0 0 4 3 Sp = Spring O = Open Ruaniaceae 0 0 0 0 0 0 0 0 0 0 0 0 0 0.000221577 0 7.73E‐05 2 0 Su = Summer C = Covered Thermoactinomycetaceae 0 0 0.000267 0.000116 0 0.00037092 0.000529 0.000526 0 0.000342922 0.000271 0.000197 0 0 0 0 8 5 F = Fall L = Lagoon Beutenbergiaceae 0.000226334 0.000331246 0.000579 0.001663 0.000620176 0 0.000106 0.000807 0.002320743 0.000571537 0.000723 0.000591 0.000169731 0 0.000606 0 13 7 W = Winter H = House Thermoanaerobacterales Family III. -

Molecular Analyses of Changes Induced in the Microbial Populations of Murine Colon After As(III) Exposure Rishu Dheer
Duquesne University Duquesne Scholarship Collection Electronic Theses and Dissertations Summer 2011 Molecular Analyses of Changes Induced in the Microbial Populations of Murine Colon After As(III) Exposure Rishu Dheer Follow this and additional works at: https://dsc.duq.edu/etd Recommended Citation Dheer, R. (2011). Molecular Analyses of Changes Induced in the Microbial Populations of Murine Colon After As(III) Exposure (Doctoral dissertation, Duquesne University). Retrieved from https://dsc.duq.edu/etd/484 This Immediate Access is brought to you for free and open access by Duquesne Scholarship Collection. It has been accepted for inclusion in Electronic Theses and Dissertations by an authorized administrator of Duquesne Scholarship Collection. For more information, please contact [email protected]. MOLECULAR ANALYSES OF CHANGES INDUCED IN THE MICROBIAL POPULATIONS OF MURINE COLON AFTER As (III) EXPOSURE A Dissertation Submitted to Bayer School of Natural and Environmental Sciences Duquesne University In partial fulfillment of the requirements for the degree of Doctor of Philosophy By Rishu Dheer August, 2011 Copyright by Rishu Dheer 2011 MOLECULAR ANALYSES OF CHANGES INDUCED IN THE MICROBIAL POPULATIONS OF MURINE COLON AFTER As (III) EXPOSURE By Rishu Dheer Approved June 14, 2011 ________________________________ ________________________________ Dr. John F. Stolz Dr. Aaron Barchowsky Director, Center of Environmental Associate Professor of Biology Research and Education (Committee Member) Professor of Biology (Committee Chair) ________________________________ -

Oral Sub-Chronic Ochratoxin a Exposure Induces Gut Microbiota Alterations in Mice
toxins Article Oral Sub-Chronic Ochratoxin a Exposure Induces Gut Microbiota Alterations in Mice María Izco 1 , Ariane Vettorazzi 2,3 , Maria de Toro 4, Yolanda Sáenz 5 and Lydia Alvarez-Erviti 1,* 1 Laboratory of Molecular Neurobiology, Center for Biomedical Research of La Rioja (CIBIR), 26006 Logroño, Spain; [email protected] 2 MITOX Group, Department of Pharmacology and Toxicology, Faculty of Pharmacy, Universidad de Navarra, 31008 Pamplona, Spain; [email protected] 3 IdiSNA, Navarra Institute for Health Research, 31008 Pamplona, Spain 4 Center for Biomedical Research of La Rioja (CIBIR), Genomics and Bioinformatics Core Facility, 26006 Logroño, Spain; [email protected] 5 Molecular Microbiology Area, Center for Biomedical Research of La Rioja (CIBIR), 26006 Logroño, Spain; [email protected] * Correspondence: [email protected]; Tel.: +34-941-278-875 Abstract: Gut microbiota plays crucial roles in maintaining host health. External factors, such as diet, medicines, and environmental toxins, influence the composition of gut microbiota. Ochratoxin A (OTA) is one of the most prevalent and relevant mycotoxins and is a highly abundant food and animal feed contaminant. In the present study, we aimed to investigate OTA gut microbiome toxicity in mice sub-chronically exposed to low doses of OTA (0.21, 0.5, and 1.5 mg/kg body weight) by daily oral gavage for 28 days. Fecal microbiota from control and OTA-treated mice was analyzed using 16S ribosomal RNA (rRNA) gene sequencing followed by metagenomics. OTA exposure caused marked changes in gut microbial community structure, including the decrease in the diversity of fecal microbiota and the relative abundance of Firmicutes, as well as the increase in the relative abundance of Bacteroidetes at the phylum level. -

Galápagos Natural History Extravaganza
GALÁPAGOS NATURAL HISTORY EXTRAVAGANZA 21 NOVEMBER – 02 DECEMBER 2021 2022 (DATES ON REQUEST) 29 OCTOBER – 09 NOVEMBER 2023 Red-footed Booby is one of three booby species likely to be found on this trip. www.birdingecotours.com [email protected] 2 | ITINERARY Galapagos: Natural History Extravaganza Just 483 years ago the first man stepped onto the Galápagos Islands and marveled at this living laboratory. Today we continue to be awestruck by this constantly changing archipelago. As the Nazca Plate moves and new islands are formed, evolution is illustrated up close and personal. Visiting the Galápagos archipelago is a dream for all naturalists. From Quito we will fly to the island of Baltra. We then will make our way to our home for the next eight days, the Samba, a spacious and luxuriously designed motor sailing yacht with wide open and shaded sun decks, a fully stocked bar, and a library. The abundant marine life that visits these waters year-round, the Marine Iguanas that rule the rocky coastlines, and of course a unique group of birds make it easy to understand why this trip is a must for birders and natural history buffs. Some of the Galápagos specials that we hope to find on this trip include Galapagos Penguin, Waved Albatross, Galapagos Shearwater, Wedge-rumped and Elliot’s Storm Petrels, Magnificent Frigatebird, Nazca, Red-footed, and Blue-footed Boobies, Lava Heron, Galapagos Hawk, Lava Gull, Galapagos Martin, Galapagos Flycatcher, Vermilion Flycatcher, Galapagos, Floreana, San Cristobal, and Espanola Mockingbirds, Vegetarian Finch, Woodpecker Finch, Common Cactus Finch, Green Warbler-Finch, Large and Small Tree Finches, Small and Medium Ground Finches, and Mangrove Finch.