Cenozoic Climate Change and Diversification on the Continental
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(Approx) Mixed Micro Shells (22G Bags) Philippines € 10,00 £8,64 $11,69 Each 22G Bag Provides Hours of Fun; Some Interesting Foraminifera Also Included
Special Price £ US$ Family Genus, species Country Quality Size Remarks w/o Photo Date added Category characteristic (€) (approx) (approx) Mixed micro shells (22g bags) Philippines € 10,00 £8,64 $11,69 Each 22g bag provides hours of fun; some interesting Foraminifera also included. 17/06/21 Mixed micro shells Ischnochitonidae Callistochiton pulchrior Panama F+++ 89mm € 1,80 £1,55 $2,10 21/12/16 Polyplacophora Ischnochitonidae Chaetopleura lurida Panama F+++ 2022mm € 3,00 £2,59 $3,51 Hairy girdles, beautifully preserved. Web 24/12/16 Polyplacophora Ischnochitonidae Ischnochiton textilis South Africa F+++ 30mm+ € 4,00 £3,45 $4,68 30/04/21 Polyplacophora Ischnochitonidae Ischnochiton textilis South Africa F+++ 27.9mm € 2,80 £2,42 $3,27 30/04/21 Polyplacophora Ischnochitonidae Stenoplax limaciformis Panama F+++ 16mm+ € 6,50 £5,61 $7,60 Uncommon. 24/12/16 Polyplacophora Chitonidae Acanthopleura gemmata Philippines F+++ 25mm+ € 2,50 £2,16 $2,92 Hairy margins, beautifully preserved. 04/08/17 Polyplacophora Chitonidae Acanthopleura gemmata Australia F+++ 25mm+ € 2,60 £2,25 $3,04 02/06/18 Polyplacophora Chitonidae Acanthopleura granulata Panama F+++ 41mm+ € 4,00 £3,45 $4,68 West Indian 'fuzzy' chiton. Web 24/12/16 Polyplacophora Chitonidae Acanthopleura granulata Panama F+++ 32mm+ € 3,00 £2,59 $3,51 West Indian 'fuzzy' chiton. 24/12/16 Polyplacophora Chitonidae Chiton tuberculatus Panama F+++ 44mm+ € 5,00 £4,32 $5,85 Caribbean. 24/12/16 Polyplacophora Chitonidae Chiton tuberculatus Panama F++ 35mm € 2,50 £2,16 $2,92 Caribbean. 24/12/16 Polyplacophora Chitonidae Chiton tuberculatus Panama F+++ 29mm+ € 3,00 £2,59 $3,51 Caribbean. -

ABSTRACT Title of Dissertation: PATTERNS IN
ABSTRACT Title of Dissertation: PATTERNS IN DIVERSITY AND DISTRIBUTION OF BENTHIC MOLLUSCS ALONG A DEPTH GRADIENT IN THE BAHAMAS Michael Joseph Dowgiallo, Doctor of Philosophy, 2004 Dissertation directed by: Professor Marjorie L. Reaka-Kudla Department of Biology, UMCP Species richness and abundance of benthic bivalve and gastropod molluscs was determined over a depth gradient of 5 - 244 m at Lee Stocking Island, Bahamas by deploying replicate benthic collectors at five sites at 5 m, 14 m, 46 m, 153 m, and 244 m for six months beginning in December 1993. A total of 773 individual molluscs comprising at least 72 taxa were retrieved from the collectors. Analysis of the molluscan fauna that colonized the collectors showed overwhelmingly higher abundance and diversity at the 5 m, 14 m, and 46 m sites as compared to the deeper sites at 153 m and 244 m. Irradiance, temperature, and habitat heterogeneity all declined with depth, coincident with declines in the abundance and diversity of the molluscs. Herbivorous modes of feeding predominated (52%) and carnivorous modes of feeding were common (44%) over the range of depths studied at Lee Stocking Island, but mode of feeding did not change significantly over depth. One bivalve and one gastropod species showed a significant decline in body size with increasing depth. Analysis of data for 960 species of gastropod molluscs from the Western Atlantic Gastropod Database of the Academy of Natural Sciences (ANS) that have ranges including the Bahamas showed a positive correlation between body size of species of gastropods and their geographic ranges. There was also a positive correlation between depth range and the size of the geographic range. -

Download Full Article 1.6MB .Pdf File
Memoirs of the National Museum of Victoria https://doi.org/10.24199/j.mmv.1962.25.10 ADDITIONS TO MARINE MOLLUSCA 177 1 May 1962 ADDITIONS TO THE MARINE MOLLUSCAN FAUNA OF SOUTH EASTERN AUSTRALIA INCLUDING DESCRIPTIONS OF NEW GENUS PILLARGINELLA, SIX NEW SPECIES AND TWO SUBSPECIES. Charles J. Gabriel, Honorary Associate in Gonchology, National Museum of Victoria. Introduction. It has always been my conviction that the spasmodic and haphazard collecting so far undertaken has not exhausted the molluscan species to be found in the deeper waters of South- eastern Australia. Only two large single collections have been made; first by the vessel " Challenger " in 1874 at Station 162 off East Moncoeur Island in 38 fathoms. These collections were described in the " Challenger " reports by Rev. Boog. Watson (Gastropoda) and E. A. Smith (Pelecypoda). In the latter was included a description of a shell Thracia watsoni not since taken in Victoria though dredged by Mr. David Howlett off St. Francis Island, South Australia. In 1910 the F. I. S. " Endeavour " made a number of hauls both north and south of Gabo Island and off Cape Everard. The u results of this collecting can be found in the Endeavour " reports. T. Iredale, 1924, published the results of shore and dredging collections made by Roy Bell. Since this time continued haphazard collecting has been carried out mostly as a hobby by trawler fishermen either for their own interest or on behalf of interested friends. Although some of this material has reached the hands of competent workers, over the years the recording of new species has probably been delayed. -

Claude Vllvens
C. VlLVENS Novapex 10 (3): 69-96. 10 octobre 2009 New species and new records of Solariellidae (Gastropoda: Trochoidea) from Indonesia and Taiwan MOy L/BRarv Claude VlLVENS Rue de Hermalle, 1 13 - B-4680 Oupeye, Belgium OCT 2 9 2002 vilvens.claude(a skynet.be Harvard KEYWORDS. Gastropoda. Solariellidae. Indonesia. Taiwan. Solariella, ArchiminoliaUtSiW/^aAiy Microgaza, Bathymophila, Spectamen. new species. ABSTRACT. New records of 8 Solariellidae species from Indonesia and Taiwan area are documented. which extend the distribution area of a number of them. 10 new species are described and compared with similar species : Solariella chodon n. sp.: S. euteia n. sp.; S. plakhus n. sp.; S. chani n. sp.: Archiminolia ptykte n. sp.; A. ostreion n. sp.; A. strobilos n. sp.; Microgaza konos n. sp.; Bathymophila aages n. sp.; Spectamen babylonia n. sp. A short conchological characterization is proposed for each genus Solariella, Archiminolia. Microgaza. Bathymophila. Spectamen. Zetela and Minolta. RESUME. De nouveaux relevés de 8 espèces de Solariellidae provenant d'Indonésie et de Taïwan sont listés, étendant ainsi l'aire de distribution d'un certain nombre d'entre elles. 10 nouvelles espèces sont décrites et comparées avec des espèces similaires : Solariella chodon n. sp.; S. euteia n. sp.: S. plakhus n. sp.; S. chani n. sp.; Archiminolia ptykte n. sp.; A. ostreion n. sp.; A. strobilos n. sp.; Microgaza konos n. sp.; Bathymophila aages n. sp.; Spectamen babylonia n. sp. Une courte caractérisation conchyliologique est proposée pour chaque genre Solariella. Archiminolia. Microgaza. Bathymophila. Spectamen. Zetela and Minolta. INTRODUCTION described accurately some species. other récent "Solariella" species is an intriguing group of authors mentioned only a few (or even no) solarielline Trochoidea. -

Anatomy of Zetela Alphonsi Vilvens, 2002 Casts Doubt on Its Original Placement Based on Conchological Characters
SPIXIANA 40 2 161-170 München, Dezember 2017 ISSN 0341-8391 Anatomy of Zetela alphonsi Vilvens, 2002 casts doubt on its original placement based on conchological characters (Mollusca, Solariellidae) Enrico Schwabe, Martin Heß, Lauren Sumner-Rooney & Javier Sellanes Schwabe, E., Heß, M., Sumner-Rooney, L. H. & Sellanes, J. 2017. Anatomy of Zetela alphonsi Vilvens, 2002 casts doubt on its original placement based on con- chological characters (Mollusca, Solariellidae). Spixiana 40 (2): 161-170. Zetela is a genus of marine gastropods belonging to the family Solariellidae (Vetigastropoda, Trochoidea). The species Zetela alphonsi Vilvens, 2002 was origi- nally described using classical conchological characters from samples obtained from deep water off Chiloé, Chile, but no other features were recorded in the original description. In the present paper, taxonomically relevant characters such as the operculum, radula, and soft part anatomy are illustrated and described for the first time from a specimen of Zetela alphonsi from a new locality, a methane seep area off the coast of central Chile. We find that many features differ substantially from other members of the genus, including several which deviate from characters that are diagnostic for Zetela. Zetela alphonsi also lacks retinal screening pigment, giving the appearance of eyelessness from gross examination. Although pigmenta- tion loss has been reported in other deep sea genera, this feature has not been re- ported in any other members of Zetela. We reconstructed a tomographic model of the eye and eyestalk, and demonstrate that a vestigial eye is still present but exter- nally invisible due to the loss of pigmentation. Based on these new morphological characters, particularly observations of the radula, we suggest that the current placement of this species in the genus Zetela should be reconsidered. -

An Annotated Checklist of the Marine Macroinvertebrates of Alaska David T
NOAA Professional Paper NMFS 19 An annotated checklist of the marine macroinvertebrates of Alaska David T. Drumm • Katherine P. Maslenikov Robert Van Syoc • James W. Orr • Robert R. Lauth Duane E. Stevenson • Theodore W. Pietsch November 2016 U.S. Department of Commerce NOAA Professional Penny Pritzker Secretary of Commerce National Oceanic Papers NMFS and Atmospheric Administration Kathryn D. Sullivan Scientific Editor* Administrator Richard Langton National Marine National Marine Fisheries Service Fisheries Service Northeast Fisheries Science Center Maine Field Station Eileen Sobeck 17 Godfrey Drive, Suite 1 Assistant Administrator Orono, Maine 04473 for Fisheries Associate Editor Kathryn Dennis National Marine Fisheries Service Office of Science and Technology Economics and Social Analysis Division 1845 Wasp Blvd., Bldg. 178 Honolulu, Hawaii 96818 Managing Editor Shelley Arenas National Marine Fisheries Service Scientific Publications Office 7600 Sand Point Way NE Seattle, Washington 98115 Editorial Committee Ann C. Matarese National Marine Fisheries Service James W. Orr National Marine Fisheries Service The NOAA Professional Paper NMFS (ISSN 1931-4590) series is pub- lished by the Scientific Publications Of- *Bruce Mundy (PIFSC) was Scientific Editor during the fice, National Marine Fisheries Service, scientific editing and preparation of this report. NOAA, 7600 Sand Point Way NE, Seattle, WA 98115. The Secretary of Commerce has The NOAA Professional Paper NMFS series carries peer-reviewed, lengthy original determined that the publication of research reports, taxonomic keys, species synopses, flora and fauna studies, and data- this series is necessary in the transac- intensive reports on investigations in fishery science, engineering, and economics. tion of the public business required by law of this Department. -

Marine Invertebrates in Tubes of Ceriantharia (Cnidaria: Anthozoa)
Biodiversity Data Journal 8: e47019 doi: 10.3897/BDJ.8.e47019 Research Article Knock knock, who’s there?: marine invertebrates in tubes of Ceriantharia (Cnidaria: Anthozoa) Hellen Ceriello‡,§, Celine S.S. Lopes‡,§, James Davis Reimer|, Torkild Bakken ¶, Marcelo V. Fukuda#, Carlo Magenta Cunha¤, Sérgio N. Stampar‡,§ ‡ Universidade Estadual Paulista "Júlio de Mesquita Filho" (UNESP), FCL, Assis, Brazil § Universidade Estadual Paulista "Júlio de Mesquita Filho" (UNESP), Instituto de Biociências, Botucatu, Brazil | University of the Ryukyus, Nishihara, Okinawa, Japan ¶ Norwegian University of Science and Technology, NTNU University Museum, Trondheim, Norway # Museu de Zoologia da Universidade de São Paulo (MZSP), São Paulo, Brazil ¤ Universidade Federal de São Paulo (Unifesp), Instituto do Mar, Santos, Brazil Corresponding author: Hellen Ceriello ([email protected]) Academic editor: Pavel Stoev Received: 02 Oct 2019 | Accepted: 04 Dec 2019 | Published: 08 Jan 2020 Citation: Ceriello H, Lopes CS.S, Reimer JD, Bakken T, Fukuda MV, Cunha CM, Stampar SN (2020) Knock knock, who’s there?: marine invertebrates in tubes of Ceriantharia (Cnidaria: Anthozoa). Biodiversity Data Journal 8: e47019. https://doi.org/10.3897/BDJ.8.e47019 Abstract This study reports on the fauna found in/on tubes of 10 species of Ceriantharia and discusses the characteristics of these occurrences, as well as the use of mollusc shells in ceriantharian tube construction. A total of 22 tubes of Ceriantharia from Argentina, Brazil, Japan, Norway, Portugal and the United States were analysed, revealing 58 species of marine invertebrates using them as alternative substrates. Based on a literature review and analyses of the sampled material, we report new occurrences for Photis sarae (Crustacea), Microgaza rotella (Mollusca), Brada sp., Dipolydora spp., Notocirrus spp., and Syllis garciai (Annelida). -

Invertebrate Fauna of Korea
Invertebrate Fauna of Korea Volume 19, Number 4 Mollusca: Gastropoda: Vetigastropoda, Sorbeoconcha Gastropods III 2017 National Institute of Biological Resources Ministry of Environment, Korea Invertebrate Fauna of Korea Volume 19, Number 4 Mollusca: Gastropoda: Vetigastropoda, Sorbeoconcha Gastropods III Jun-Sang Lee Kangwon National University Invertebrate Fauna of Korea Volume 19, Number 4 Mollusca: Gastropoda: Vetigastropoda, Sorbeoconcha Gastropods III Copyright ⓒ 2017 by the National Institute of Biological Resources Published by the National Institute of Biological Resources Environmental Research Complex, Hwangyeong-ro 42, Seo-gu Incheon 22689, Republic of Korea www.nibr.go.kr All rights reserved. No part of this book may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording, or otherwise, without the prior permission of the National Institute of Biological Resources. ISBN : 978-89-6811-266-9 (96470) ISBN : 978-89-94555-00-3 (세트) Government Publications Registration Number : 11-1480592-001226-01 Printed by Junghaengsa, Inc. in Korea on acid-free paper Publisher : Woonsuk Baek Author : Jun-Sang Lee Project Staff : Jin-Han Kim, Hyun Jong Kil, Eunjung Nam and Kwang-Soo Kim Published on February 7, 2017 The Flora and Fauna of Korea logo was designed to represent six major target groups of the project including vertebrates, invertebrates, insects, algae, fungi, and bacteria. The book cover and the logo were designed by Jee-Yeon Koo. Chlorococcales: 1 Preface The biological resources include all the composition of organisms and genetic resources which possess the practical and potential values essential to human live. Biological resources will be firmed competition of the nation because they will be used as fundamental sources to make highly valued products such as new lines or varieties of biological organisms, new material, and drugs. -
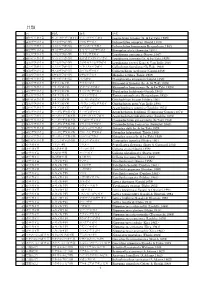
貝類 目 科名 和名 学名 1 新ヒザラガイ目 サメハダヒザラガイ科 サメハダヒザラガイ Leptochiton Hirasei (Is
貝類 目 科名 和名 学名 1 新ヒザラガイ目 サメハダヒザラガイ科 サメハダヒザラガイ Leptochiton hirasei (Is. & Iw.Taki,1929) 2 新ヒザラガイ目 ウスヒザラガイ科 ウスヒザラガイ Ischnochiton comptus (Gould,1859) 3 新ヒザラガイ目 ウスヒザラガイ科 ホソウスヒザラガイ Ischnochiton boninensis Bergenhayn,1933 4 新ヒザラガイ目 ウスヒザラガイ科 オオセスジヒザラガイ Stenoplax alata (Sowerby,1841) 5 新ヒザラガイ目 ウスヒザラガイ科 ヤスリヒザラガイ Lepidozona coreanica (Reeve,1847) 6 新ヒザラガイ目 ウスヒザラガイ科 セダカヤスリヒザラガイ Lepidozona iyoensis (Is. & Iw.Taki,1929) 7 新ヒザラガイ目 ウスヒザラガイ科 ミガキヤスリヒザラガイ Lepidozona reevei Kaas & Van Belle,1987 8 新ヒザラガイ目 ウスヒザラガイ科 ハチノスヒザラガイ Callochiton foveolatus (Is.Taki,1938) 9 新ヒザラガイ目 ウスヒザラガイ科 カブトヒザラガイ Callistochiton jacobaeus (Gould,1859) 10 新ヒザラガイ目 ヒゲヒザラガイ科 ヒゲヒザラガイ Mopalia retifera Thiele,1909 11 新ヒザラガイ目 ヒゲヒザラガイ科 ババガセ Placiphorella stimpsoni (Gould,1859) 12 新ヒザラガイ目 クサズリガイ科 クサズリガイ Rhyssoplax kurodai (Is. & Iw.Taki,1929) 13 新ヒザラガイ目 クサズリガイ科 オオクサズリガイ Rhyssoplax komaianus (Is. & Iw.Taki,1929) 14 新ヒザラガイ目 クサズリガイ科 ナミジワヒザラガイ Tegulaplax hululensis (Smith,1903) 15 新ヒザラガイ目 クサズリガイ科 アヤヒザラガイ Tonicia interplicata (Bergenhayn,1933) 16 新ヒザラガイ目 クサズリガイ科 ニシキヒザラガイ Onithochiton hirasei Pilsbry,1901 17 新ヒザラガイ目 クサズリガイ科 ゴトウニシキヒザラガイ Onithochiton gotoi Van Belle,1993 18 新ヒザラガイ目 クサズリガイ科 ヒザラガイ Acanthopleura japonica (Lischke,1873) 19 新ヒザラガイ目 ケハダヒザラガイ科 ケハダヒザラガイ Acanthochiton defilippii (Tapparone-Canefri,1874) 20 新ヒザラガイ目 ケハダヒザラガイ科 ヒメケハダヒザラガイ Acanthochiton rubrolineatus (Lischke,1873) 21 新ヒザラガイ目 ケハダヒザラガイ科 ミツカドヒザラガイ Craspedochiton pyramidalis (Is.Taki,1938) 22 新ヒザラガイ目 ケハダヒザラガイ科 フチドリヒザラガイ Craspedochiton laqueatus (Sowerby,1841) 23 新ヒザラガイ目 ケハダヒザラガイ科 ウスベニヒザラガイ Notoplax dalli Is. & Iw.Taki,1929 24 新ヒザラガイ目 -

New Records and New Species of Calliotropis (Gastropoda
C. VILVENS N o v a p e x 7 (2-3): 55-71, 10 juin 2006 New records and new species ofCalliotropis (Gastropoda: Chilodontidae: Calliotropinae) from Madagascar, Mayotte Island and Reunion Island Claude VILVENS Rue de Hermalle, 113 - B-4680 Oupeye, Belgium [email protected] KEYWORDS. Gastropoda, Chilodontidae, Madagascar, Mayotte, Reunion,Calliotropis n. sp., Trochidae. ABSTRACT. New records ofCalliotropis species from the western Indian Ocean are listed. Some Indo-Pacific species are recorded for the first time in the Madagascar area.Calliotropis velata n. sp., C. ericius n. sp., C. bucina n. sp., C. babylonia n. sp., andC. solariellaformis n. sp. are described and compared with similarCalliotropis species. RESUME. De nouveaux relevés de Calliotropis provenant de l'Océan Indien occidental sont listés. Des espèces décrites de l'Indo-Pacifique sont enregistrées pour la première fois dans la région de Madagascar.Calliotropis velata n. sp., C. ericius n. sp., C. bucina n. sp., C. babylonia n. sp. et C. solariellaformis n. sp. sont décrites et comparées avec des espèces analogues de Calliotropis. INTRODUCTION and Zululand areas (Kilbum & Herbert, 1994); part of this material was studied by Kilbum (e.g. 1973, 1977) The deep-water fauna of the western and south and Herbert (e.g. 1987, 1991, 1992, 1993). western parts of the Indian Ocean remains poorly The present paper is based on deep-water material known. The German Valdivia expedition (1898-99)from the Madagascar region brought together in carried a number of deep-water operations off what MNHN from various sources. Surveys for deep-water was then German East Africa; the gastropods were shrimp fisheries were carried out in 1971-73 on R.V. -

Marrying Molecules and Morphology: First Steps Towards a Reevaluation Of
Journal of The Malacological Society of London Molluscan Studies Journal of Molluscan Studies (2020) 86: 1–26. doi:10.1093/mollus/eyz038 Advance Access Publication Date: 9 March 2020 Featured Article Marrying molecules and morphology: first steps towards a reevaluation of solariellid genera (Gastropoda: Trochoidea) in the light of molecular phylogenetic studies S. T. Williams1, Y. K a n o 2, A. Warén3,4,5 and D. G. Herbert6 Downloaded from https://academic.oup.com/mollus/article/86/1/1/5716161 by guest on 30 September 2021 1Department of Life Sciences, Natural History Museum, Cromwell Rd, London SW7 5BD, UK; 2Atmosphere and Ocean Research Institute, The University of Tokyo, 5-1-5 Kashiwanoha, Kashiwa, Chiba 277-8564, Japan; 3Swedish Museum of Natural History, Box 50007, SE-10405 Stockholm, Sweden; 4Lomvägen 30, SE-19256 Sollentuna, Sweden; 5c/- Jägmarker, Timmerbacken, Norra Lundby, SE-53293 Axvall, Sweden; and 6Department of Natural Sciences, National Museum of Wales, Cathays Park, Cardiff CF10 3NP,UK Correspondence: S.T. Williams; e-mail: [email protected] (Received 14 June 2019; editorial decision 2 November 2019) ABSTRACT The assignment of species to the vetigastropod genus Solariella Wood, 1842, and therefore the family Solariellidae Powell, 1951, is complicated by the fact that the type species (Solariella maculata Wood, 1842) is a fossil described from the Upper Pliocene. Assignment of species to genera has proved difficult in the past, and the type genus has sometimes acted as a ‘wastebasket’ for species that cannot easily be referred to another genus. In the light of a new systematic framework provided by two recent publications presenting the first molecular phylogenetic data for the group, we reassess the shell characters that are most useful for delimiting genera. -

Abbreviation Kiel S. 2005, New and Little Known Gastropods from the Albian of the Mahajanga Basin, Northwestern Madagaskar
1 Reference (Explanations see mollusca-database.eu) Abbreviation Kiel S. 2005, New and little known gastropods from the Albian of the Mahajanga Basin, Northwestern Madagaskar. AF01 http://www.geowiss.uni-hamburg.de/i-geolo/Palaeontologie/ForschungImadagaskar.htm (11.03.2007, abstract) Bandel K. 2003, Cretaceous volutid Neogastropoda from the Western Desert of Egypt and their place within the noegastropoda AF02 (Mollusca). Mitt. Geol.-Paläont. Inst. Univ. Hamburg, Heft 87, p 73-98, 49 figs., Hamburg (abstract). www.geowiss.uni-hamburg.de/i-geolo/Palaeontologie/Forschung/publications.htm (29.10.2007) Kiel S. & Bandel K. 2003, New taxonomic data for the gastropod fauna of the Uzamba Formation (Santonian-Campanian, South AF03 Africa) based on newly collected material. Cretaceous research 24, p. 449-475, 10 figs., Elsevier (abstract). www.geowiss.uni-hamburg.de/i-geolo/Palaeontologie/Forschung/publications.htm (29.10.2007) Emberton K.C. 2002, Owengriffithsius , a new genus of cyclophorid land snails endemic to northern Madagascar. The Veliger 45 (3) : AF04 203-217. http://www.theveliger.org/index.html Emberton K.C. 2002, Ankoravaratra , a new genus of landsnails endemic to northern Madagascar (Cyclophoroidea: Maizaniidae?). AF05 The Veliger 45 (4) : 278-289. http://www.theveliger.org/volume45(4).html Blaison & Bourquin 1966, Révision des "Collotia sensu lato": un nouveau sous-genre "Tintanticeras". Ann. sci. univ. Besancon, 3ème AF06 série, geologie. fasc.2 :69-77 (Abstract). www.fossile.org/pages-web/bibliographie_consacree_au_ammon.htp (20.7.2005) Bensalah M., Adaci M., Mahboubi M. & Kazi-Tani O., 2005, Les sediments continentaux d'age tertiaire dans les Hautes Plaines AF07 Oranaises et le Tell Tlemcenien (Algerie occidentale).