A Spatially Explicit Nanomechanical Model of the Half-Sarcomere: Myofilament Compliance Affects Ca2+-Activation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Passive Tension in Cardiac Muscle: Contribution of Collagen, Titin, Microtubules, and Intermediate Filaments
Biophysical Journal Volume 68 March 1995 1027-1044 1027 Passive Tension in Cardiac Muscle: Contribution of Collagen, Titin, Microtubules, and Intermediate Filaments Henk L. Granzier and Thomas C. Irving Department of Veterinary and Comparative Anatomy, Pharmacology, and Physiology, Washington State University, Pullman, Washington 99164·6520 USA ABSTRACT The passive tension-sarcomere length relation of rat cardiac muscle was investigated by studying passive (or not activated) single myocytes and trabeculae. The contribution ofcollagen, titin, microtubules, and intermediate filaments to tension and stiffness was investigated by measuring (1) the effects of KCI/KI extraction on both trabeculae and single myocytes, (2) the effect of trypsin digestion on single myocytes, and (3) the effect of colchicine on single myocytes. It was found that over the working range of sarcomeres in the heart (lengths -1.9-2.2 11m), collagen and titin are the most important contributors to passive tension with titin dominating at the shorter end of the working range and collagen at longer lengths. Microtubules made a modest contribution to passive tension in some cells, but on average their contribution was not significant. Finally, intermediate filaments contribl,!ted about 10%to passive tension oftrabeculae at sarcomere lengths from -1.9to 2.1 11m, and theircontribution dropped to only a few percent at longer lengths. At physiological sarcomere lengths of the heart, cardiac titin developed much higher tensions (>20-fold) than did skeletal muscle titin at comparable lengths. This might be related to the finding that cardiac titin has a molecular mass of 2.5 MDa, 0.3-0.5 MDa smaller than titin of mammalian skeletal muscle, which is predicted to result in a much shorter extensible titin segment in the I-band of cardiac muscle. -
THE MUSCLE SPINDLE Anatomical Structures of the Spindle Apparatus
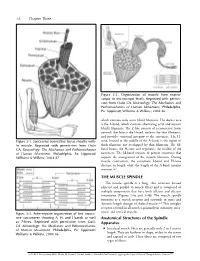
56 Chapter Three Figure 3-2. Organization of muscle from macro- scopic to microscopic levels. Reprinted with permis- sion from Oatis CA. Kinesiology: The Mechanics and Pathomechanics of Human Movement. Philadelphia, Pa: Lippincott Williams & Wilkins; 2004:46. which contains only actin (thin) filaments. The darker area is the A-band, which contains alternating actin and myosin (thick) filaments. The Z-line consists of a connective tissue network that bisects the I-band, anchors the thin filaments, and provides structural integrity to the sarcomere. The H- Figure 3-1. Successive connective tissue sheaths with- zone, located in the middle of the A-band, is the region of in muscle. Reprinted with permission from Oatis thick filaments not overlapped by thin filaments. The M- CA. Kinesiology: The Mechanics and Pathomechanics band bisects the H-zone and represents the middle of the of Human Movement. Philadelphia, Pa: Lippincott sarcomere. The M-band consists of protein structures that Williams & Wilkins; 2004:47. support the arrangement of the myosin filaments. During muscle contraction, the sarcomere I-band and H-zone decrease in length while the length of the A-band remains constant.2,3 THE MUSCLE SPINDLE The muscle spindle is a long, thin structure located adjacent and parallel to muscle fibers and is composed of multiple components that have both afferent and efferent innervation (Figures 3-4a and 3-4b). The muscle spindle functions as a stretch receptor and responds to static and dynamic length changes of skeletal muscle.4-6 This complex receptor is found in all muscles, primarily in extremity, inter- costal, and cervical muscles. -

Uniform Sarcomere Shortening Behavior in Isolated Cardiac Muscle Cells
Uniform Sarcomere Shortening Behavior in Isolated Cardiac Muscle Cells JOHN W. KRUEGER, DAMIAN FORLETTI, and BEATRICE A. WITTENBERG From the Albert Einstein College of Medicine, Departments of Medicine and Physiology, Bronx, New York 10461 ABSTRACT We have observed the dynamics of sarcomere shortening and the diffracting action of single, functionally intact, unattached cardiac muscle cells enzymatically isolated from the ventricular tissue of adult rats. Sarcomere length was measured either (a) continuously by a light diffraction method or (b) by direct inspection of the cell's striated image as recorded on videotape or by cinemicroscopy (120-400 frames/s). At physiological levels of added CaCl2 (0.5- 2.0 mM), many cells were quiescent (i.e., they did not beat spontaneously) and contracted in response to electrical stimulation (~ 1.0-ms pulse width). Sarcomere length in the quiescent, unstimulated cells (1.93 + 0.10 [SD] /~m), at peak shortening (1.57 + 0.13/~m, n = 49), and the maximum velocity of sarcomere shortening and relengthening were comparable to previous observations in intact heart muscle preparations. The dispersion of light diffracted by the cell remained narrow, and individual striations remained distinct and laterally well registered throughout the shortening-relengthening cycle. In contrast, apprecia- ble nonuniformity and internal buckling were seen at sarcomere lengths < 1.8 /~m when the resting cell, embedded in gelatin, was longitudinally compressed. These results indicate (a) that shortening and relengthening is characterized by uniform activation between myofibrils within the cardiac cell and (b) that physiologically significant relengthening forces in living heart muscle originate at the level of the cell rather than in extracellular connections. -

Titin N2A Domain and Its Interactions at the Sarcomere
International Journal of Molecular Sciences Review Titin N2A Domain and Its Interactions at the Sarcomere Adeleye O. Adewale and Young-Hoon Ahn * Department of Chemistry, Wayne State University, Detroit, MI 48202, USA; [email protected] * Correspondence: [email protected]; Tel.: +1-(313)-577-1384 Abstract: Titin is a giant protein in the sarcomere that plays an essential role in muscle contraction with actin and myosin filaments. However, its utility goes beyond mechanical functions, extending to versatile and complex roles in sarcomere organization and maintenance, passive force, mechanosens- ing, and signaling. Titin’s multiple functions are in part attributed to its large size and modular structures that interact with a myriad of protein partners. Among titin’s domains, the N2A element is one of titin’s unique segments that contributes to titin’s functions in compliance, contraction, structural stability, and signaling via protein–protein interactions with actin filament, chaperones, stress-sensing proteins, and proteases. Considering the significance of N2A, this review highlights structural conformations of N2A, its predisposition for protein–protein interactions, and its multiple interacting protein partners that allow the modulation of titin’s biological effects. Lastly, the nature of N2A for interactions with chaperones and proteases is included, presenting it as an important node that impacts titin’s structural and functional integrity. Keywords: titin; N2A domain; protein–protein interaction 1. Introduction Citation: Adewale, A.O.; Ahn, Y.-H. The complexity of striated muscle is defined by the intricate organization of its com- Titin N2A Domain and Its ponents [1]. The involuntary cardiac and voluntary skeletal muscles are the primary types Interactions at the Sarcomere. -

Effect of Camkiiδ Deletion on Sarcomere Structure
Features Efect of CaMKIIδ Deletion on Sarcomere Structure Tik-Chee (Jenny) Cheng, Sukriti Dewan, Andrew McCulloch, Jef Omens Jenny Cheng is a senior at Earl Warren College majoring in Bioengineering with a focus in Biotechnology. In Fall 2015, she will be pursuing her PhD in Biomedical Engineering at the University of Wisconsin-Madison, studying cardiovascular pathophysiology for clinical applications. Heart failure, a progressive cardiomyopathy, a condition with condition that is typically enlarged left ventricles and/or undetected and often dilated ventricular walls that can misdiagnosed, is the leading lead to dysfunction and inadequate cause of deaths around pumping of blood to the rest of the world. The disorder is the body. Calcium/calmodulin- CaMKII isoform (CaMKIIδ) characterized by the inability dependent protein kinase II appear to be protected of the heart to keep up with (CaMKII) is a protein kinase from such dysfunction and the body’s demand for blood that can phosphorylate many hypertrophy. circulation. It is initiated substrates and regulates a wide by structural and functional range of cellular function. Its role Normal myocardial function changes from in calcium signaling is dependent on the structure The disorder is molecular to pathways has been of cardiomyocytes, specifcally systemic levels characterized by implicated in the the basic subunits of muscle that result in the inability of the pathophysiology called sarcomeres. Sarcomeres compensatory cardiac pump to of adverse cardiac contract and generate force physiological keep up with the left ventricular by utilizing the motor changes, body’s demand. remodeling. movements of thin and thick commonly Animal models flaments, specifcally myosin termed cardiac remodeling. -

Defects in T-Tubular Electrical Activity Underlie Local Alterations of Calcium Release in Heart Failure
Defects in T-tubular electrical activity underlie local alterations of calcium release in heart failure Claudia Crocinia, Raffaele Coppinib, Cecilia Ferrantinic, Ping Yand, Leslie M. Loewd, Chiara Tesic, Elisabetta Cerbaib, Corrado Poggesic, Francesco S. Pavonea,e,f, and Leonardo Sacconia,f,1 aEuropean Laboratory for Non-Linear Spectroscopy, 50019 Florence, Italy; bDivision of Pharmacology, Department “NeuroFarBa,” University of Florence, 50139 Florence, Italy; cDivision of Physiology, Department of Experimental and Clinical Medicine, University of Florence, 50134 Florence, Italy; dR. D. Berlin Center for Cell Analysis and Modeling, University of Connecticut Health Center, Farmington, CT 06030; eDepartment of Physics and Astronomy, University of Florence, 50019 Sesto Fiorentino, Italy; and fNational Institute of Optics, National Research Council, 50125 Florence, Italy Edited by Clara Franzini-Armstrong, University of Pennsylvania Medical Center, Philadelphia, PA, and approved September 15, 2014 (received for review June 20, 2014) Action potentials (APs), via the transverse axial tubular system in a rat model of postischemic HF, structurally remodeled TATS + (TATS), synchronously trigger uniform Ca2 release throughout the exhibits abnormal electrical activity, i.e., failure of AP propagation cardiomyocyte. In heart failure (HF), TATS structural remodeling and presence of local spontaneous depolarizations. Tubular AP occurs, leading to asynchronous Ca2+ release across the myocyte failures and spontaneous activity can potentially aggravate asyn- + and contributing to contractile dysfunction. In cardiomyocytes from chronous Ca2 release and determine nonhomogeneous myofibril + failing rat hearts, we previously documented the presence of TATS contraction. Simultaneous recording of local Ca2 release and AP in elements which failed to propagate AP and displayed spontaneous the tubular network is needed to unravel the consequences of these + + electrical activity; the consequence for Ca2 release remained, how- electrical anomalies on intracellular Ca2 dynamics. -

Microfluidic Perfusion Shows Intersarcomere Dynamics Within Single Skeletal Muscle Myofibrils
Microfluidic perfusion shows intersarcomere dynamics within single skeletal muscle myofibrils Felipe de Souza Leitea, Fabio C. Minozzoa, David Altmanb, and Dilson E. Rassiera,c,d,1 aDepartment of Kinesiology and Physical Education, McGill University, Montreal, QC, Canada, H2W 1S4; bDepartment of Physics, Willamette University, Salem, OR 97301; cDepartment of Physiology, McGill University, Montreal, QC, Canada, H3G 1Y6; and dDepartment of Physics, McGill University, Montreal, QC, Canada H3A 2T8 Edited by James A. Spudich, Stanford University School of Medicine, Stanford, CA, and approved July 5, 2017 (received for review January 30, 2017) The sarcomere is the smallest functional unit of myofibrils in passive force production, which enables the myofibrils to stabilize in striated muscles. Sarcomeres are connected in series through a a given activation condition. Because of technical limitations, the network of elastic and structural proteins. During myofibril activa- mechanisms governing the interactionofsarcomeresinamyofibril tion, sarcomeres develop forces that are regulated through complex and its consequences for force production remain unclear (11). dynamics among their structures. The mechanisms that regulate In this study, we tested the hypotheses that the mechanical work intersarcomere dynamics are unclear, which limits our understand- of one sarcomere effectively communicates with other sarcomeres ing of fundamental muscle features. Such dynamics are associated in series through the passive work of titin and that myofibril me- with the loss in forces caused by mechanical instability encountered chanics are largely governed by intersarcomere dynamics. To test in muscle diseases and cardiomyopathy and may underlie potential these hypotheses, we used microfluidic perfusions to locally control target treatments for such conditions. In this study, we developed a one sarcomere or a predetermined group of sarcomeres within an microfluidic perfusion system to control one sarcomere within a isolated myofibril. -

The Sarcomere Force–Length Relationship in an Intact Muscle–Tendon Unit Eng Kuan Moo, Timothy R
© 2020. Published by The Company of Biologists Ltd | Journal of Experimental Biology (2020) 223, jeb215020. doi:10.1242/jeb.215020 RESEARCH ARTICLE The sarcomere force–length relationship in an intact muscle–tendon unit Eng Kuan Moo, Timothy R. Leonard and Walter Herzog* ABSTRACT muscle level, structural and mechanical complexities are added The periodic striation pattern in skeletal muscle reflects the length of (Blemker et al., 2005; Heemskerk et al., 2005; Lovering et al., the basic contractile unit: the sarcomere. More than half a century ago, 2013). The most important of these is that the line of action of force Gordon, Huxley and Julian provided strong support for the ‘sliding production in a muscle is typically not along the fibre direction as filament’ theory through experiments with single muscle fibres. The pressure, shear forces and extracellular matrix stiffness contribute to sarcomere force–length (FL) relationship has since been extrapolated whole-muscle forces in a way not possible in a single fibre (Azizi to whole muscles in an attempt to unravel in vivo muscle function. et al., 2008; Gillies and Lieber, 2011; Holt et al., 2016; Maas et al., However, these extrapolations were frequently associated with non- 2001; Purslow, 2008; Raiteri et al., 2018). trivial assumptions, such as muscle length changes corresponding At present, our understanding of the FL relationship at the whole- linearly to SL changes. Here, we determined the in situ sarcomere muscle level has been derived primarily from experiments in which FL relationship in a whole muscle preparation by simultaneously muscle forces were measured at varying muscle lengths, without in situ measuring muscle force and individual SLs in an intact muscle–tendon direct measurement of sarcomere length (SL) (Gokhin et al., unit (MTU) using state-of-the-art multi-photon excitation microscopy. -

Zooming in on Muscle Cells Has Produced the First High-Resolution 3D Image of the Sarcomere 24 March 2021
Zooming in on muscle cells has produced the first high-resolution 3D image of the sarcomere 24 March 2021 frozen muscle," says Raunser. Electron cryo-tomography was for a long time an established yet niche methodology. But recent technical advances in electron cryo-microscopy as well as the new development of cryo focused ion beam (FIB) milling are pushing electron cryo- tomography resolution. Similar to electron cryo- microscopy, researchers flash-freeze the biological sample at a very low temperature (- 175 °C). Through this process, the sample preserves its hydration and fine structure and remains close to its 3D-reconstruction of the sarcomere. Coloured strains native state. FIB milling is then applied to shave show individual filaments. Credit: MPI of Molecular away extra material and obtain an ideal thickness Physiology of around 100 nanometers for the transmission electron microscope, which acquires multiple images as the sample is tilted along an axis. Finally, computational methods reconstruct a three- An international team, led by Stefan Raunser, dimensional picture at high resolution. Director at the Max Planck Institute of Molecular Physiology in Dortmund, in collaboration with Raunser's team performed electron cryo- Mathias Gautel at the King's College in London, tomography on mouse myofibrils isolated at the has produced the first high-resolution 3D image of King's College, and obtained a resolution of one the sarcomere, the basic contractile unit of skeletal nanometer (a millionth of a millimeter, enough to and heart muscle cells, by using electron cryo- see fine structures within a protein): "We can now tomography. Electron cryo-tomography capability look at a myofibril with details thought unimaginable of imaging structures directly in frozen muscle cells only four years ago. -

The Unified Myofibrillar Matrix for Force Generation in Muscle
ARTICLE https://doi.org/10.1038/s41467-020-17579-6 OPEN The unified myofibrillar matrix for force generation in muscle ✉ T. Bradley Willingham1, Yuho Kim 1, Eric Lindberg1, Christopher K. E. Bleck 1 & Brian Glancy 1,2 Human movement occurs through contraction of the basic unit of the muscle cell, the sarcomere. Sarcomeres have long been considered to be arranged end-to-end in series along the length of the muscle into tube-like myofibrils with many individual, parallel myofibrils 1234567890():,; comprising the bulk of the muscle cell volume. Here, we demonstrate that striated muscle cells form a continuous myofibrillar matrix linked together by frequently branching sarco- meres. We find that all muscle cells contain highly connected myofibrillar networks though the frequency of sarcomere branching goes down from early to late postnatal development and is higher in slow-twitch than fast-twitch mature muscles. Moreover, we show that the myofibrillar matrix is united across the entire width of the muscle cell both at birth and in mature muscle. We propose that striated muscle force is generated by a singular, mesh-like myofibrillar network rather than many individual, parallel myofibrils. 1 National Heart, Lung and Blood Institute, National Institutes of Health, Bethesda, MD 20892, USA. 2 National Institute of Arthritis and Musculoskeletal and ✉ Skin Diseases, National Institutes of Health, Bethesda, MD 20892, USA. email: [email protected] NATURE COMMUNICATIONS | (2020) 11:3722 | https://doi.org/10.1038/s41467-020-17579-6 | www.nature.com/naturecommunications 1 ARTICLE NATURE COMMUNICATIONS | https://doi.org/10.1038/s41467-020-17579-6 he mechanisms underlying how muscle contraction gen- muscle cell structures for abnormalities. -

Sarcomeric Gene Variants and Their Role with Left Ventricular Dysfunction in Background of Coronary Artery Disease
biomolecules Review Sarcomeric Gene Variants and Their Role with Left Ventricular Dysfunction in Background of Coronary Artery Disease 1, 2, , 2, Surendra Kumar y, Vijay Kumar * y and Jong-Joo Kim * 1 Department of Anatomy, All India Institute of Medical Sciences, New Delhi 110029, India; [email protected] 2 Department of Biotechnology, Yeungnam University, Gyeongsan, Gyeongbuk 38541, Korea * Correspondence: [email protected] (V.K.); [email protected] (J.-J.K.); Tel.: +82-53-810-3027 or +82-10-9668-3464 (J.-J.K.); Fax: +82-53-801-3464 (J.-J.K.) These authors contributed equally to this work. y Received: 1 March 2020; Accepted: 11 March 2020; Published: 12 March 2020 Abstract: Cardiovascular diseases are one of the leading causes of death in developing countries, generally originating as coronary artery disease (CAD) or hypertension. In later stages, many CAD patients develop left ventricle dysfunction (LVD). Left ventricular ejection fraction (LVEF) is the most prevalent prognostic factor in CAD patients. LVD is a complex multifactorial condition in which the left ventricle of the heart becomes functionally impaired. Various genetic studies have correlated LVD with dilated cardiomyopathy (DCM). In recent years, enormous progress has been made in identifying the genetic causes of cardiac diseases, which has further led to a greater understanding of molecular mechanisms underlying each disease. This progress has increased the probability of establishing a specific genetic diagnosis, and thus providing new opportunities for practitioners, patients, and families to utilize this genetic information. A large number of mutations in sarcomeric genes have been discovered in cardiomyopathies. In this review, we will explore the role of the sarcomeric genes in LVD in CAD patients, which is a major cause of cardiac failure and results in heart failure. -

Extra- and Intrafusal Muscle Fibre Type Compositions of the Human Masseter at Young Age in Perspective of Growth and Functional
Extra- and intrafusal muscle fibre type compositions of the human masseter at young age In perspective of growth and functional maturation of the jaw-face motor system Catharina Österlund Departments of Odontology, Clinical Oral Physiology, and Integrative Medical Biology, Section for Anatomy, Umeå University, SE-901 87 Umeå, Sweden Umeå 2011 Responsible publisher under swedish law: the Dean of the Medical Faculty This work is protected by the Swedish Copyright Legislation (Act 1960:729) ISBN: 978-91-7459-285-6. ISSN: 0345-7532. Series No 120. Cover image: Muscle cross-section from young masseter stained by monoclonal antibody MHCn against myosin heavy chain-fetal, showing extrafusal muscle fibres, and intrafusal muscle fibres within a muscle spindle. Note above, muscle spindle containing numerous intrafusal fibres. Variability in staining intesity reflect variable physiological properties of fibres. Elektronisk version tillgänglig på http://umu.diva-portal.org/ Tryck/Printed by: Print & Media, Umeå Universitet Umeå, Sweden, 2011 Gud ge mig sinnesro att acceptera det jag inte kan förändra, mod att förändra det jag kan och förstånd att inse skillnaden. Till min familj TABLE OF CONTENTS Table of contents i Abstract iii Abbreviations iv Svensk sammanfattning v Original papers vi BACKGROUND 1 INTRODUCTION 1 Muscles and muscle fibres 1 Sarcomeric myosin 5 Muscle plasticity 6 Muscle development 7 Jaw functions 8 Jaw-face growth 8 Masseter muscle 10 Biceps brachii muscle 13 Muscle spindles 14 Myofascial pain 18 Purpose of the thesis 19 AIMS