Diverse and Complex Muscle Spindle Afferent Firing Properties Emerge
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

VIEW Open Access Muscle Spindle Function in Healthy and Diseased Muscle Stephan Kröger* and Bridgette Watkins
Kröger and Watkins Skeletal Muscle (2021) 11:3 https://doi.org/10.1186/s13395-020-00258-x REVIEW Open Access Muscle spindle function in healthy and diseased muscle Stephan Kröger* and Bridgette Watkins Abstract Almost every muscle contains muscle spindles. These delicate sensory receptors inform the central nervous system (CNS) about changes in the length of individual muscles and the speed of stretching. With this information, the CNS computes the position and movement of our extremities in space, which is a requirement for motor control, for maintaining posture and for a stable gait. Many neuromuscular diseases affect muscle spindle function contributing, among others, to an unstable gait, frequent falls and ataxic behavior in the affected patients. Nevertheless, muscle spindles are usually ignored during examination and analysis of muscle function and when designing therapeutic strategies for neuromuscular diseases. This review summarizes the development and function of muscle spindles and the changes observed under pathological conditions, in particular in the various forms of muscular dystrophies. Keywords: Mechanotransduction, Sensory physiology, Proprioception, Neuromuscular diseases, Intrafusal fibers, Muscular dystrophy In its original sense, the term proprioception refers to development of head control and walking, an early im- sensory information arising in our own musculoskeletal pairment of fine motor skills, sensory ataxia with un- system itself [1–4]. Proprioceptive information informs steady gait, increased stride-to-stride variability in force us about the contractile state and movement of muscles, and step length, an inability to maintain balance with about muscle force, heaviness, stiffness, viscosity and ef- eyes closed (Romberg’s sign), a severely reduced ability fort and, thus, is required for any coordinated move- to identify the direction of joint movements, and an ab- ment, normal gait and for the maintenance of a stable sence of tendon reflexes [6–12]. -

Muscle Tissue
10 Muscle Tissue PowerPoint® Lecture Presentations prepared by Jason LaPres Lone Star College—North Harris © 2012 Pearson Education, Inc. 10-1 An Introduction to Muscle Tissue • Learning Outcomes • 10-1 Specify the functions of skeletal muscle tissue. • 10-2 Describe the organization of muscle at the tissue level. • 10-3 Explain the characteristics of skeletal muscle fibers, and identify the structural components of a sarcomere. • 10-4 Identify the components of the neuromuscular junction, and summarize the events involved in the neural control of skeletal muscle contraction and relaxation. © 2012 Pearson Education, Inc. 10-1 An Introduction to Muscle Tissue • Learning Outcomes • 10-5 Describe the mechanism responsible for tension production in a muscle fiber, and compare the different types of muscle contraction. • 10-6 Describe the mechanisms by which muscle fibers obtain the energy to power contractions. • 10-7 Relate the types of muscle fibers to muscle performance, and distinguish between aerobic and anaerobic endurance. © 2012 Pearson Education, Inc. 10-1 An Introduction to Muscle Tissue • Learning Outcomes • 10-8 Identify the structural and functional differences between skeletal muscle fibers and cardiac muscle cells. • 10-9 Identify the structural and functional differences between skeletal muscle fibers and smooth muscle cells, and discuss the roles of smooth muscle tissue in systems throughout the body. © 2012 Pearson Education, Inc. An Introduction to Muscle Tissue • Muscle Tissue • A primary tissue type, divided into: • Skeletal muscle tissue • Cardiac muscle tissue • Smooth muscle tissue © 2012 Pearson Education, Inc. 10-1 Functions of Skeletal Muscle Tissue • Skeletal Muscles • Are attached to the skeletal system • Allow us to move • The muscular system • Includes only skeletal muscles © 2012 Pearson Education, Inc. -

Interpretation of Sensory Information from Skeletal Muscle Receptors for External Control Milan Djilas
Interpretation of Sensory Information From Skeletal Muscle Receptors For External Control Milan Djilas To cite this version: Milan Djilas. Interpretation of Sensory Information From Skeletal Muscle Receptors For External Control. Automatic. Université Montpellier II - Sciences et Techniques du Languedoc, 2008. English. tel-00333530 HAL Id: tel-00333530 https://tel.archives-ouvertes.fr/tel-00333530 Submitted on 23 Oct 2008 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. UNIVERSITE MONTPELLIER II SCIENCES ET TECHNIQUES DU LANGUEDOC T H E S E pour obtenir le grade de DOCTEUR DE L'UNIVERSITE MONTPELLIER II Formation doctorale: SYSTEMES AUTOMATIQUES ET MICROELECTRONIQUES Ecole Doctorale: INFORMATION, STRUCTURES ET SYSTEMES présentée et soutenue publiquement par Milan DJILAS le 13 octobre 2008 Titre: INTERPRETATION DES INFORMATIONS SENSORIELLES DES RECEPTEURS DU MUSCLE SQUELETTIQUE POUR LE CONTROLE EXTERNE INTERPRETATION OF SENSORY INFORMATION FROM SKELETAL MUSCLE RECEPTORS FOR EXTERNAL CONTROL JURY Jacques LEVY VEHEL Directeur de Recherches, INRIA Rapporteur -

Characterization of Cecal Smooth Muscle Contraction in Laying Hens
veterinary sciences Communication Characterization of Cecal Smooth Muscle Contraction in Laying Hens Katrin Röhm 1, Martin Diener 2 , Korinna Huber 1 and Jana Seifert 1,* 1 Institute of Animal Science, University of Hohenheim, 70593 Stuttgart, Germany; [email protected] (K.R.); [email protected] (K.H.) 2 Institute of Veterinary Physiology and Biochemistry, Justus-Liebig University, 35392 Gießen, Germany; [email protected] * Correspondence: [email protected] Abstract: The ceca play an important role in the physiology of the gastrointestinal tract in chickens. Nevertheless, there is a gap of knowledge regarding the functionality of the ceca in poultry, especially with respect to physiological cecal smooth muscle contraction. The aim of the current study is the ex vivo characterization of cecal smooth muscle contraction in laying hens. Muscle strips of circular cecal smooth muscle from eleven hens are prepared to investigate their contraction ex vivo. Contraction is detected using an isometric force transducer, determining its frequency, height and intensity. Spontaneous contraction of the chicken cecal smooth muscle and the influence of buffers (calcium-free buffer and potassium-enriched buffer) and drugs (carbachol, nitroprusside, isoprenaline and Verapamil) affecting smooth muscle contraction at different levels are characterized. A decrease in smooth muscle contraction is observed when a calcium-free buffer is used. Carbachol causes an increase in smooth muscle contraction, whereas atropine inhibits contraction. Nitroprusside, isoprenaline and Verapamil result in a depression of smooth muscle contraction. In conclusion, the present results confirm a similar contraction behavior of cecal smooth muscles in laying hens as Citation: Röhm, K.; Diener, M.; shown previously in other species. -

Passive Tension in Cardiac Muscle: Contribution of Collagen, Titin, Microtubules, and Intermediate Filaments
Biophysical Journal Volume 68 March 1995 1027-1044 1027 Passive Tension in Cardiac Muscle: Contribution of Collagen, Titin, Microtubules, and Intermediate Filaments Henk L. Granzier and Thomas C. Irving Department of Veterinary and Comparative Anatomy, Pharmacology, and Physiology, Washington State University, Pullman, Washington 99164·6520 USA ABSTRACT The passive tension-sarcomere length relation of rat cardiac muscle was investigated by studying passive (or not activated) single myocytes and trabeculae. The contribution ofcollagen, titin, microtubules, and intermediate filaments to tension and stiffness was investigated by measuring (1) the effects of KCI/KI extraction on both trabeculae and single myocytes, (2) the effect of trypsin digestion on single myocytes, and (3) the effect of colchicine on single myocytes. It was found that over the working range of sarcomeres in the heart (lengths -1.9-2.2 11m), collagen and titin are the most important contributors to passive tension with titin dominating at the shorter end of the working range and collagen at longer lengths. Microtubules made a modest contribution to passive tension in some cells, but on average their contribution was not significant. Finally, intermediate filaments contribl,!ted about 10%to passive tension oftrabeculae at sarcomere lengths from -1.9to 2.1 11m, and theircontribution dropped to only a few percent at longer lengths. At physiological sarcomere lengths of the heart, cardiac titin developed much higher tensions (>20-fold) than did skeletal muscle titin at comparable lengths. This might be related to the finding that cardiac titin has a molecular mass of 2.5 MDa, 0.3-0.5 MDa smaller than titin of mammalian skeletal muscle, which is predicted to result in a much shorter extensible titin segment in the I-band of cardiac muscle. -

Tissue Engineered Myelination and the Stretch Reflex Arc Sensory Circuit: Defined Medium Ormulation,F Interface Design and Microfabrication
University of Central Florida STARS Electronic Theses and Dissertations, 2004-2019 2009 Tissue Engineered Myelination And The Stretch Reflex Arc Sensory Circuit: Defined Medium ormulation,F Interface Design And Microfabrication John Rumsey University of Central Florida Part of the Biology Commons Find similar works at: https://stars.library.ucf.edu/etd University of Central Florida Libraries http://library.ucf.edu This Doctoral Dissertation (Open Access) is brought to you for free and open access by STARS. It has been accepted for inclusion in Electronic Theses and Dissertations, 2004-2019 by an authorized administrator of STARS. For more information, please contact [email protected]. STARS Citation Rumsey, John, "Tissue Engineered Myelination And The Stretch Reflex Arc Sensory Circuit: Defined Medium Formulation, Interface Design And Microfabrication" (2009). Electronic Theses and Dissertations, 2004-2019. 3826. https://stars.library.ucf.edu/etd/3826 TISSUE ENGINEERED MYELINATION AND THE STRETCH REFLEX ARC SENSORY CIRCUIT: DEFINED MEDIUM FORMULATION, INTERFACE DESIGN AND MICROFABRICATION by JOHN WAYNE RUMSEY B.S. University of Florida, 2001 M.S. University of Central Florida, 2004 A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Burnett School of Biomedical Sciences in the College of Medicine at the University of Central Florida Orlando, Florida Fall Term 2009 Major Professor: James J. Hickman ABSTRACT The overall focus of this research project was to develop an in vitro tissue- engineered system that accurately reproduced the physiology of the sensory elements of the stretch reflex arc as well as engineer the myelination of neurons in the systems. In order to achieve this goal we hypothesized that myelinating culture systems, intrafusal muscle fibers and the sensory circuit of the stretch reflex arc could be bioengineered using serum-free medium formulations, growth substrate interface design and microfabrication technology. -

Back-To-Basics: the Intricacies of Muscle Contraction
Back-to- MIOTA Basics: The CONFERENCE OCTOBER 11, Intricacies 2019 CHERI RAMIREZ, MS, of Muscle OTRL Contraction OBJECTIVES: 1.Review the anatomical structure of a skeletal muscle. 2.Review and understand the process and relationship between skeletal muscle contraction with the vital components of the nervous system, endocrine system, and skeletal system. 3.Review the basic similarities and differences between skeletal muscle tissue, smooth muscle tissue, and cardiac muscle tissue. 4.Review the names, locations, origins, and insertions of the skeletal muscles found in the human body. 5.Apply the information learned to enhance clinical practice and understanding of the intricacies and complexity of the skeletal muscle system. 6.Apply the information learned to further educate clients on the importance of skeletal muscle movement, posture, and coordination in the process of rehabilitation, healing, and functional return. 1. Epithelial Four Basic Tissue Categories 2. Muscle 3. Nervous 4. Connective A. Loose Connective B. Bone C. Cartilage D. Blood Introduction There are 3 types of muscle tissue in the muscular system: . Skeletal muscle: Attached to bones of skeleton. Voluntary. Striated. Tubular shape. Cardiac muscle: Makes up most of the wall of the heart. Involuntary. Striated with intercalated discs. Branched shape. Smooth muscle: Found in walls of internal organs and walls of vascular system. Involuntary. Non-striated. Spindle shape. 4 Structure of a Skeletal Muscle Skeletal Muscles: Skeletal muscles are composed of: • Skeletal muscle tissue • Nervous tissue • Blood • Connective tissues 5 Connective Tissue Coverings Connective tissue coverings over skeletal muscles: .Fascia .Tendons .Aponeuroses 6 Fascia: Definition: Layers of dense connective tissue that separates muscle from adjacent muscles, by surrounding each muscle belly. -
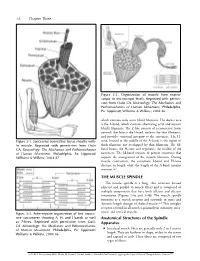
THE MUSCLE SPINDLE Anatomical Structures of the Spindle Apparatus
56 Chapter Three Figure 3-2. Organization of muscle from macro- scopic to microscopic levels. Reprinted with permis- sion from Oatis CA. Kinesiology: The Mechanics and Pathomechanics of Human Movement. Philadelphia, Pa: Lippincott Williams & Wilkins; 2004:46. which contains only actin (thin) filaments. The darker area is the A-band, which contains alternating actin and myosin (thick) filaments. The Z-line consists of a connective tissue network that bisects the I-band, anchors the thin filaments, and provides structural integrity to the sarcomere. The H- Figure 3-1. Successive connective tissue sheaths with- zone, located in the middle of the A-band, is the region of in muscle. Reprinted with permission from Oatis thick filaments not overlapped by thin filaments. The M- CA. Kinesiology: The Mechanics and Pathomechanics band bisects the H-zone and represents the middle of the of Human Movement. Philadelphia, Pa: Lippincott sarcomere. The M-band consists of protein structures that Williams & Wilkins; 2004:47. support the arrangement of the myosin filaments. During muscle contraction, the sarcomere I-band and H-zone decrease in length while the length of the A-band remains constant.2,3 THE MUSCLE SPINDLE The muscle spindle is a long, thin structure located adjacent and parallel to muscle fibers and is composed of multiple components that have both afferent and efferent innervation (Figures 3-4a and 3-4b). The muscle spindle functions as a stretch receptor and responds to static and dynamic length changes of skeletal muscle.4-6 This complex receptor is found in all muscles, primarily in extremity, inter- costal, and cervical muscles. -

Muscle Physiology Dr
Muscle Physiology Dr. Ebneshahidi Copyright © 2004 Pearson Education, Inc., publishing as Benjamin Cummings Skeletal Muscle Figure 9.2 (a) Copyright © 2004 Pearson Education, Inc., publishing as Benjamin Cummings Functions of the muscular system . 1. Locomotion . 2. Vasoconstriction and vasodilatation- constriction and dilation of blood vessel Walls are the results of smooth muscle contraction. 3. Peristalsis – wavelike motion along the digestive tract is produced by the Smooth muscle. 4. Cardiac motion . 5. Posture maintenance- contraction of skeletal muscles maintains body posture and muscle tone. 6. Heat generation – about 75% of ATP energy used in muscle contraction is released as heat. Copyright. © 2004 Pearson Education, Inc., publishing as Benjamin Cummings . Striation: only present in skeletal and cardiac muscles. Absent in smooth muscle. Nucleus: smooth and cardiac muscles are uninculcated (one nucleus per cell), skeletal muscle is multinucleated (several nuclei per cell ). Transverse tubule ( T tubule ): well developed in skeletal and cardiac muscles to transport calcium. Absent in smooth muscle. Intercalated disk: specialized intercellular junction that only occurs in cardiac muscle. Control: skeletal muscle is always under voluntary control‚ with some exceptions ( the tongue and pili arrector muscles in the dermis). smooth and cardiac muscles are under involuntary control. Copyright © 2004 Pearson Education, Inc., publishing as Benjamin Cummings Innervation: motor unit . a) a motor nerve and a myofibril from a neuromuscular junction where gap (called synapse) occurs between the two structures. at the end of motor nerve‚ neurotransmitter (i.e. acetylcholine) is stored in synaptic vesicles which will release the neurotransmitter using exocytosis upon the stimulation of a nerve impulse. Across the synapse the surface the of myofibril contains receptors that can bind with the neurotransmitter. -

Single-Cell Analysis Uncovers Fibroblast Heterogeneity
ARTICLE https://doi.org/10.1038/s41467-020-17740-1 OPEN Single-cell analysis uncovers fibroblast heterogeneity and criteria for fibroblast and mural cell identification and discrimination ✉ Lars Muhl 1,2 , Guillem Genové 1,2, Stefanos Leptidis 1,2, Jianping Liu 1,2, Liqun He3,4, Giuseppe Mocci1,2, Ying Sun4, Sonja Gustafsson1,2, Byambajav Buyandelger1,2, Indira V. Chivukula1,2, Åsa Segerstolpe1,2,5, Elisabeth Raschperger1,2, Emil M. Hansson1,2, Johan L. M. Björkegren 1,2,6, Xiao-Rong Peng7, ✉ Michael Vanlandewijck1,2,4, Urban Lendahl1,8 & Christer Betsholtz 1,2,4 1234567890():,; Many important cell types in adult vertebrates have a mesenchymal origin, including fibro- blasts and vascular mural cells. Although their biological importance is undisputed, the level of mesenchymal cell heterogeneity within and between organs, while appreciated, has not been analyzed in detail. Here, we compare single-cell transcriptional profiles of fibroblasts and vascular mural cells across four murine muscular organs: heart, skeletal muscle, intestine and bladder. We reveal gene expression signatures that demarcate fibroblasts from mural cells and provide molecular signatures for cell subtype identification. We observe striking inter- and intra-organ heterogeneity amongst the fibroblasts, primarily reflecting differences in the expression of extracellular matrix components. Fibroblast subtypes localize to discrete anatomical positions offering novel predictions about physiological function(s) and regulatory signaling circuits. Our data shed new light on the diversity of poorly defined classes of cells and provide a foundation for improved understanding of their roles in physiological and pathological processes. 1 Karolinska Institutet/AstraZeneca Integrated Cardio Metabolic Centre, Blickagången 6, SE-14157 Huddinge, Sweden. -

Emphasizing Task-Specific Hypertrophy to Enhance Sequential Strength and Power Performance
Journal of Functional Morphology and Kinesiology Review Emphasizing Task-Specific Hypertrophy to Enhance Sequential Strength and Power Performance S. Kyle Travis 1,* , Ai Ishida 1 , Christopher B. Taber 2 , Andrew C. Fry 3 and Michael H. Stone 1 1 Center of Excellence for Sport Science and Coach Education, Department of Sport, Exercise, Recreation, and Kinesiology, East Tennessee State University, Johnson City, TN 37604, USA; [email protected] (A.I.); [email protected] (M.H.S.) 2 Department of Physical Therapy and Human Movement Science, Sacred Heart University, Fairfield, CT 06825, USA; [email protected] 3 Jayhawk Athletic Performance Laboratory, Department of Health, Sport, and Exercise Sciences, University of Kansas, Lawrence, KS 66046, USA; [email protected] * Correspondence: [email protected] Received: 20 August 2020; Accepted: 21 October 2020; Published: 27 October 2020 Abstract: While strength is indeed a skill, most discussions have primarily considered structural adaptations rather than ultrastructural augmentation to improve performance. Altering the structural component of the muscle is often the aim of hypertrophic training, yet not all hypertrophy is equal; such alterations are dependent upon how the muscle adapts to the training stimuli and overall training stress. When comparing bodybuilders to strength and power athletes such as powerlifters, weightlifters, and throwers, while muscle size may be similar, the ability to produce force and power is often inequivalent. Thus, performance differences go beyond structural changes and may be due to the muscle’s ultrastructural constituents and training induced adaptations. Relative to potentiating strength and power performances, eliciting specific ultrastructural changes should be a variable of interest during hypertrophic training phases. -

Be Able to Describe a Stretch Reflex
be able to describe a stretch reflex .(1) (2) Define muscle tone (3) be able to explain what is muscle tone (4) describe the structure , innervations and function of the muscle spindle . (5) explain what is meant by static and dynamic stretch reflex . (6) describe the spinal and supraspinal regulation of the stretch reflex . (7) describe the inverse stretch reflex and its function Stretch reflex: Whenever a muscle is stretched suddenly, excitation of the muscle spindle causes reflex contraction of the large muscle fiber (See the pictures). It is deep and monosynaptic reflex. Stretch response is produced by co-activation of alpha & gamma motor neurons. But it is maintained mainly by the tonic ( continuous) discharge of Gamma Efferent neurons. Muscle spindle: It is a sensory receptors which are distributed throughout the muscle and it sends information to nervous system about muscle length or rate of change in muscle length. - Each spindle is built around 3-12 intrafusal muscle fibers. - Muscle spindles are parallel to extrafusal muscle fibers and they are attached to them or to the tendon. Types of Intrafusal fibers Each intrafusal fiber consists of: (1) Central non-contractile area (receptor 2) Nuclear chain fibers: area). thinner and shorter 1) Nuclear bag fibers: than nuclear bag fibers (2) Peripheral contractile area. contain many nuclei in , and have one line of a dilated central area ( “ nuclei spread in a chain bag ” ) . Typically there along the receptor area are 2 nuclear bag fibers . There are 4 – 9 per spindle . nuclear chain