Structure and Function of Muscle Fibers and Motor Units
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1933.Full.Pdf
0270.6474/84/0408-1933$02.00/O The Journal of Neuroscience Copyright 0 Society for Neuroscience Vol. 4, No. 8, pp. 1933-1943 Printed in U.S.A. August 1984 PARTIAL PURIFICATION AND FUNCTIONAL IDENTIFICATION OF A CALMODULIN-ACTIVATED, ADENOSINE 5’-TRIPHOSPHATE-DEPENDENT CALCIUM PUMP FROM SYNAPTIC PLASMA MEMBRANES1 DIANE M. PAPAZIAN,* HANNAH RAHAMIMOFF,$ AND STANLEY M. GOLDIN§’ Departments of *Biological Chemistry and $Pharmacology, Harvard Medical School, Boston, Massachusetts 02115 and *Department of Biochemistry, Hadassah Medical School, Hebrew University, Jerusalem, Israel Received July 18, 1983; Revised February 27, 1984; Accepted February 28, 1984 Abstract Synaptic plasma membranes isolated from rat brain contain a calmodulin-activated Ca2+ pump. It has been purified 80- to 160-fold by solubilization with Triton X-100 and affinity chromatography on a calmodulin- Sepharose 4B column. After reconstitution into phospholipid vesicles, the affinity-purified pump efficiently catalyzed ATP dependent Ca2+ transport, which was activated 7- to g-fold by calmodulin. The major protein component of the affinity-purified preparation had a M, = 140,000; it was virtually the only band visualized on a Coomassie blue-stained SDS polyacrylamide gel. It has been identified as the Ca”’ pump by two functional criteria. First, it was phosphorylated by [y-““P]ATP in a Ca’+-dependent manner; the phosphorylated protein had the chemical reactivity of an acyl phosphate, characteristic of the phosphorylated intermediates of ion-transporting ATPases. Second, the protein was enriched by transport-specific fractiona- tion, a density gradient procedure which uses the transport properties of the reconstituted Ca2+ pump as a physical tool for its purification. -

VIEW Open Access Muscle Spindle Function in Healthy and Diseased Muscle Stephan Kröger* and Bridgette Watkins
Kröger and Watkins Skeletal Muscle (2021) 11:3 https://doi.org/10.1186/s13395-020-00258-x REVIEW Open Access Muscle spindle function in healthy and diseased muscle Stephan Kröger* and Bridgette Watkins Abstract Almost every muscle contains muscle spindles. These delicate sensory receptors inform the central nervous system (CNS) about changes in the length of individual muscles and the speed of stretching. With this information, the CNS computes the position and movement of our extremities in space, which is a requirement for motor control, for maintaining posture and for a stable gait. Many neuromuscular diseases affect muscle spindle function contributing, among others, to an unstable gait, frequent falls and ataxic behavior in the affected patients. Nevertheless, muscle spindles are usually ignored during examination and analysis of muscle function and when designing therapeutic strategies for neuromuscular diseases. This review summarizes the development and function of muscle spindles and the changes observed under pathological conditions, in particular in the various forms of muscular dystrophies. Keywords: Mechanotransduction, Sensory physiology, Proprioception, Neuromuscular diseases, Intrafusal fibers, Muscular dystrophy In its original sense, the term proprioception refers to development of head control and walking, an early im- sensory information arising in our own musculoskeletal pairment of fine motor skills, sensory ataxia with un- system itself [1–4]. Proprioceptive information informs steady gait, increased stride-to-stride variability in force us about the contractile state and movement of muscles, and step length, an inability to maintain balance with about muscle force, heaviness, stiffness, viscosity and ef- eyes closed (Romberg’s sign), a severely reduced ability fort and, thus, is required for any coordinated move- to identify the direction of joint movements, and an ab- ment, normal gait and for the maintenance of a stable sence of tendon reflexes [6–12]. -

Desmin- Cytoskeleton Marker Cat#: ET1606-30
rev. 04/13/16 Desmin- Cytoskeleton Marker Cat#: ET1606-30 Prod uct Type: R ecombinant r abbit mono clonal IgG, primary antibodies Species reactivity: Human, Mouse, Rat, Zebra fish Applications: WB, ICC/IF, IHC, FC Molecular Wt.: 53 kDa Description: Cytoskeletal intermediate filaments (IFs) constitute a diverse group of proteins that are expressed in a highly tissue - specific manner. IFs are constructed from two - chain α -helical coiled-coil molecules arranged on an imperfect helical Fig1: Western blot analysis of Desmin on lattice, and have been widely used as markers for distinguishing different lysates using anti-Desmin antibody at individual cell types within a tissue and identifying the origins 1/1,000 dilution. of metastatic tumors. Vimentin is an IF general marker of cells Positive control: originating in the mesenchyme. Vimentin and Desmin, a related class III IF, are both expressed during skeletal muscle development. Lane 1: Human skeletal muscle Desmin, a 469 amino acid protein found near the Z line in sarcomeres, Lane 2: Mouse heart is expressed more frequently in adult differentiated state tissues. Desmin makes up attachments between the terminal Z-disc and membrane-associated proteins to form a force-transmitting system. Mutations in the gene encoding for Desmin are associated with adult-onset skeletal myopathy, sporadic disease and mild cardiac involvement. Immunogen: Recombinant protein. Positive control: Fig2: ICC staining Desmin in C2C12 cells (green). C2C12, human uterus tissue, mouse bladder tissue, mouse heart The nuclear counter stain is DAPI (blue). Cells tissue, mouse skeletal muscle tissue. were fixed in paraformaldehyde, permeabilised with 0.25% Triton X100/PBS. -

Vocabulario De Morfoloxía, Anatomía E Citoloxía Veterinaria
Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) Servizo de Normalización Lingüística Universidade de Santiago de Compostela COLECCIÓN VOCABULARIOS TEMÁTICOS N.º 4 SERVIZO DE NORMALIZACIÓN LINGÜÍSTICA Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) 2008 UNIVERSIDADE DE SANTIAGO DE COMPOSTELA VOCABULARIO de morfoloxía, anatomía e citoloxía veterinaria : (galego-español- inglés) / coordinador Xusto A. Rodríguez Río, Servizo de Normalización Lingüística ; autores Matilde Lombardero Fernández ... [et al.]. – Santiago de Compostela : Universidade de Santiago de Compostela, Servizo de Publicacións e Intercambio Científico, 2008. – 369 p. ; 21 cm. – (Vocabularios temáticos ; 4). - D.L. C 2458-2008. – ISBN 978-84-9887-018-3 1.Medicina �������������������������������������������������������������������������veterinaria-Diccionarios�������������������������������������������������. 2.Galego (Lingua)-Glosarios, vocabularios, etc. políglotas. I.Lombardero Fernández, Matilde. II.Rodríguez Rio, Xusto A. coord. III. Universidade de Santiago de Compostela. Servizo de Normalización Lingüística, coord. IV.Universidade de Santiago de Compostela. Servizo de Publicacións e Intercambio Científico, ed. V.Serie. 591.4(038)=699=60=20 Coordinador Xusto A. Rodríguez Río (Área de Terminoloxía. Servizo de Normalización Lingüística. Universidade de Santiago de Compostela) Autoras/res Matilde Lombardero Fernández (doutora en Veterinaria e profesora do Departamento de Anatomía e Produción Animal. -

The Muscular System
THE MUSCULAR SYSTEM COMPILED BY HOWIE BAUM 1 Muscles make up the bulk of the body and account for 1/3 of its weight.!! Blood vessels and nerves run to every muscle, helping control and regulate each muscle’s function. The muscular system creates body heat and also moves the: Bones of the Skeletal system Food through Digestive system Blood through the Circulatory system Fluids through the Excretory system MUSCLE TISSUE The body has 3 main types of muscle tissue 1) Skeletal, 2) Smooth, and 3) Cardiac SKELETAL MUSCLE SMOOTH MUSCLE CARDIAC MUSCLE Skeletal muscles attach to and move bones by contracting and relaxing in response to voluntary messages from the nervous system. Skeletal muscle tissue is composed of long cells called muscle fibers that have a striated appearance. Muscle fibers are organized into bundles supplied by blood vessels and innervated by motor neurons. Muscle structure Skeletal (striated or voluntary) muscle consists of densely packed groups of hugely elongated cells known as myofibers. These are grouped into bundles (fascicles). A typical myofiber is 2–3 centimeters ( 3/4–1 1/5 in) long and 0.05millimeters (1/500 inch) in diameter and is composed of narrower structures – myofibrils. These contain thick and thin myofilaments made up mainly of the proteins actin and myosin. Numerous capillaries keep the muscle supplied with the oxygen and glucose needed to fuel contraction. Skeletal Muscles • Skeletal muscles attach to bones by tendons (connective tissue) and enable movement. • Skeletal muscles are mostly voluntary Feel the back of your ankle to feel your Achilles tendon - the largest tendon in your body. -

Passive Tension in Cardiac Muscle: Contribution of Collagen, Titin, Microtubules, and Intermediate Filaments
Biophysical Journal Volume 68 March 1995 1027-1044 1027 Passive Tension in Cardiac Muscle: Contribution of Collagen, Titin, Microtubules, and Intermediate Filaments Henk L. Granzier and Thomas C. Irving Department of Veterinary and Comparative Anatomy, Pharmacology, and Physiology, Washington State University, Pullman, Washington 99164·6520 USA ABSTRACT The passive tension-sarcomere length relation of rat cardiac muscle was investigated by studying passive (or not activated) single myocytes and trabeculae. The contribution ofcollagen, titin, microtubules, and intermediate filaments to tension and stiffness was investigated by measuring (1) the effects of KCI/KI extraction on both trabeculae and single myocytes, (2) the effect of trypsin digestion on single myocytes, and (3) the effect of colchicine on single myocytes. It was found that over the working range of sarcomeres in the heart (lengths -1.9-2.2 11m), collagen and titin are the most important contributors to passive tension with titin dominating at the shorter end of the working range and collagen at longer lengths. Microtubules made a modest contribution to passive tension in some cells, but on average their contribution was not significant. Finally, intermediate filaments contribl,!ted about 10%to passive tension oftrabeculae at sarcomere lengths from -1.9to 2.1 11m, and theircontribution dropped to only a few percent at longer lengths. At physiological sarcomere lengths of the heart, cardiac titin developed much higher tensions (>20-fold) than did skeletal muscle titin at comparable lengths. This might be related to the finding that cardiac titin has a molecular mass of 2.5 MDa, 0.3-0.5 MDa smaller than titin of mammalian skeletal muscle, which is predicted to result in a much shorter extensible titin segment in the I-band of cardiac muscle. -

Muscle Histology
Muscle Histology Dr. Heba Kalbouneh Functions of muscle tissue ▪ Movement ▪ Maintenance of posture ▪ Joint stabilization ▪ Heat generation Types of Muscle Tissue ▪ Skeletal muscle ▪ Cardiac muscle ▪ Smooth muscle Types of Muscle Tissue Skeletal •Attach to and move skeleton •40% of body weight •Fibers = multinucleate cells (embryonic cells fuse) •Cells with obvious striations •Contractions are voluntary Cardiac: only in the wall of the heart •Cells are striated •Contractions are involuntary (not voluntary) Smooth: walls of hollow organs •Lack striations •Contractions are involuntary (not voluntary) Similarities… ▪ Their cells are called fibers because they are elongated ▪ Contraction depends on myofilaments ▪ Actin ▪ Myosin ▪ Plasma membrane is called sarcolemma ▪ Sarcos = flesh ▪ Lemma = sheath SKELETAL MUSCLES Epimysium: surrounds whole muscle Endomysium is around each muscle fiber Perimysium is around fascicle = muscle cell= myofiber Skeletal muscle This big cylinder is a fiber: a cell ▪ Fibers (each is one cell) have striations ▪ Myofibrils are organelles of the cell: these are made -an organelle up of myofilaments ▪ Sarcomere ▪ Basic unit of contraction ▪ Myofibrils are long rows of repeating sarcomeres ▪ Boundaries: Z discs (or lines) Sarcomere M line provides an attachment to myosin filaments Z line provides an attachment to actin filaments A band is the darker band of the myofibril containing myosin filaments H band is the lighter section in the middle of the A band where only myosin is present I band is the lighter band containing -

Skeletal Muscle Physiology
This document was created by Alex Yartsev ([email protected]); if I have used your data or images and forgot to reference you, please email me. Skeletal Muscle Physiology First of all, which muscle is which - Skeletal muscle: o Well-developed cross-striations o Does not contract in absence of a nerve stimulus o The individual muscle fibers DO NOT connect functionally or anatomically (i.e. they don’t form a single sheet of cells, and one fiber’s action potential wont get transmitted to the next) o Generally, skeletal muscle is under voluntary control - Cardiac muscle: o Also has cross-striations o Is functionally syncytial: cells are connected well enough to conduct action potentials to one another o Can contract on its own, without stimulus (but this is under some control via the autonomic nervous system, which modulates its activity) - Smooth muscle: o Has no cross-striations o Two broad types: . VISCERAL or “unitary” smooth muscle: Functionally syncytial, action potentials propagate from cell to cell Contains pacemakers which discharge irregularly, but remains under control of the autonomic nervous system Found in most hollow viscera . MULTI-UNIT SMOOTH MUSCLE Found in the eye and some other locations Does NOT activate spontaneously SKELETAL MUSCLE ORGANIZATION - Each muscle is a bundle of fibers - Each fiber is a long, multinucleated single cell - Each fiber is surrounded by a SARCOLEMMA- the cell membrane - There are NO SYNCYTIAL BRIDGES between the cells. When one cell goes off, the others don’t follow. TRANSVERSE TUBULES: T-tubules, invaginations of SARCOLEMMA: the muscle cell membrane the sarcolemma, they form part of the T-system; the space inside is an extension of the extracellular space. -

Back-To-Basics: the Intricacies of Muscle Contraction
Back-to- MIOTA Basics: The CONFERENCE OCTOBER 11, Intricacies 2019 CHERI RAMIREZ, MS, of Muscle OTRL Contraction OBJECTIVES: 1.Review the anatomical structure of a skeletal muscle. 2.Review and understand the process and relationship between skeletal muscle contraction with the vital components of the nervous system, endocrine system, and skeletal system. 3.Review the basic similarities and differences between skeletal muscle tissue, smooth muscle tissue, and cardiac muscle tissue. 4.Review the names, locations, origins, and insertions of the skeletal muscles found in the human body. 5.Apply the information learned to enhance clinical practice and understanding of the intricacies and complexity of the skeletal muscle system. 6.Apply the information learned to further educate clients on the importance of skeletal muscle movement, posture, and coordination in the process of rehabilitation, healing, and functional return. 1. Epithelial Four Basic Tissue Categories 2. Muscle 3. Nervous 4. Connective A. Loose Connective B. Bone C. Cartilage D. Blood Introduction There are 3 types of muscle tissue in the muscular system: . Skeletal muscle: Attached to bones of skeleton. Voluntary. Striated. Tubular shape. Cardiac muscle: Makes up most of the wall of the heart. Involuntary. Striated with intercalated discs. Branched shape. Smooth muscle: Found in walls of internal organs and walls of vascular system. Involuntary. Non-striated. Spindle shape. 4 Structure of a Skeletal Muscle Skeletal Muscles: Skeletal muscles are composed of: • Skeletal muscle tissue • Nervous tissue • Blood • Connective tissues 5 Connective Tissue Coverings Connective tissue coverings over skeletal muscles: .Fascia .Tendons .Aponeuroses 6 Fascia: Definition: Layers of dense connective tissue that separates muscle from adjacent muscles, by surrounding each muscle belly. -

Structure of a Skeletal Muscle
STRUCTURE OF A SKELETAL MUSCLE Skeletal muscles are not made of muscle cells alone • Skeletal muscle contains blood vessels that supply muscle cells with oxygen and glucose, and remove wastes, and nerves that coordinate muscle contraction • 1 § Each individual muscle cell (fiber) is surrounded by the _____________ § Several muscle cells are bundled together into a _________ by the _____________ § All fascicles that make up a muscle are, in turn, enclosed by the _____________ § Interconnected connective tissues taper down and connect to tendons or other connective tissues; attach muscle to bone or other structure to be moved Figure 9.1 Position and structure of a skeletal muscle. 2 FUNCTIONS OF SKELETAL MUSCLES • Muscle contractions are involved in more than just movement of bones at a joint: § § Contraction of diaphragm muscle is a vital function associated with respiratory system § _________________ – sitting, standing, holding head upright § Skeletal muscles attached to facial skin allow for facial expression; muscles in throat assist with swallowing § Sphincters composed of skeletal muscle allow conscious control over opening and closing of body openings § Support of soft tissue – abdominal walls, pelvic floor 3 • Functional groups of muscles: generally takes cooperation of several individual muscles working as a group to perform a movement or action § __________________ provide most force for a given muscle action § _____________have opposite action of agonist; allows for modulation and control of agonist movement § _____________aid agonists by supplying supplemental force, minimizing unwanted movement, and by helping to stabilize joints § _____________also provide stabilizing force that anchors a bone; protection from injury due to unnecessary movements Figure 9.3 Functional groups of muscles. -

Evidence for the Nonmuscle Nature of The" Myofibroblast" of Granulation Tissue and Hypertropic Scar. an Immunofluorescence Study
American Journal of Pathology, Vol. 130, No. 2, February 1988 Copyright i American Association of Pathologists Evidencefor the Nonmuscle Nature ofthe "Myofibroblast" ofGranulation Tissue and Hypertropic Scar An Immunofluorescence Study ROBERT J. EDDY, BSc, JANE A. PETRO, MD, From the Department ofAnatomy, the Department ofSurgery, and JAMES J. TOMASEK, PhD Division ofPlastic and Reconstructive Surgery, and the Department of Orthopaedic Surgery, New York Medical College, Valhalla, New York Contraction is an important phenomenon in wound cell-matrix attachment in smooth muscle and non- repair and hypertrophic scarring. Studies indicate that muscle cells, respectively. Myofibroblasts can be iden- wound contraction involves a specialized cell known as tified by their intense staining of actin bundles with the myofibroblast, which has morphologic character- either anti-actin antibody or NBD-phallacidin. Myofi- istics of both smooth muscle and fibroblastic cells. In broblasts in all tissues stained for nonmuscle but not order to better characterize the myofibroblast, the au- smooth muscle myosin. In addition, nonmuscle myo- thors have examined its cytoskeleton and surrounding sin was localized as intracellular fibrils, which suggests extracellular matrix (ECM) in human burn granula- their similarity to stress fibers in cultured fibroblasts. tion tissue, human hypertrophic scar, and rat granula- The ECM around myofibroblasts stains intensely for tion tissue by indirect immunofluorescence. Primary fibronectin but lacks laminin, which suggests that a antibodies used in this study were directed against 1) true basal lamina is not present. The immunocytoche- smooth muscle myosin and 2) nonmuscle myosin, mical findings suggest that the myofibroblast is a spe- components ofthe cytoskeleton in smooth muscle and cialized nonmuscle type of cell, not a smooth muscle nonmuscle cells, respectively, and 3) laminin and 4) cell. -
THE MUSCLE SPINDLE Anatomical Structures of the Spindle Apparatus
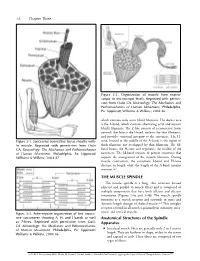
56 Chapter Three Figure 3-2. Organization of muscle from macro- scopic to microscopic levels. Reprinted with permis- sion from Oatis CA. Kinesiology: The Mechanics and Pathomechanics of Human Movement. Philadelphia, Pa: Lippincott Williams & Wilkins; 2004:46. which contains only actin (thin) filaments. The darker area is the A-band, which contains alternating actin and myosin (thick) filaments. The Z-line consists of a connective tissue network that bisects the I-band, anchors the thin filaments, and provides structural integrity to the sarcomere. The H- Figure 3-1. Successive connective tissue sheaths with- zone, located in the middle of the A-band, is the region of in muscle. Reprinted with permission from Oatis thick filaments not overlapped by thin filaments. The M- CA. Kinesiology: The Mechanics and Pathomechanics band bisects the H-zone and represents the middle of the of Human Movement. Philadelphia, Pa: Lippincott sarcomere. The M-band consists of protein structures that Williams & Wilkins; 2004:47. support the arrangement of the myosin filaments. During muscle contraction, the sarcomere I-band and H-zone decrease in length while the length of the A-band remains constant.2,3 THE MUSCLE SPINDLE The muscle spindle is a long, thin structure located adjacent and parallel to muscle fibers and is composed of multiple components that have both afferent and efferent innervation (Figures 3-4a and 3-4b). The muscle spindle functions as a stretch receptor and responds to static and dynamic length changes of skeletal muscle.4-6 This complex receptor is found in all muscles, primarily in extremity, inter- costal, and cervical muscles.