ICD-10-PCS and ICD-9-CM Procedure Presentations with Public Comment 12:30 PM – 1:30 PM Lunch Break 1:30 PM – 5:00 PM Diagnosis Presentations with Public Comment
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Urology 1 Cystoscopes
Urology 1 Cystoscopes 2 Urethrotomes 3 Resectoscopes 4 Uretero-Renoscopes 5 Nephroscopes 6 Lithotripsy (UreTron) 7 Laser Therapy 8 Small Caliber 9 Fluid Management 10 Accessories Richard Wolf Medical Instruments Corporation assumes no responsibility or liability for any errors or omissions in the content of this catalog. The information contained in this catalog is provided on an “as is” basis with no guarantees of completeness, accuracy, usefulness, or timeliness, and without warranties of any kind whatsoever, expressed or implied. 1777- 02.01-1118USA Cysto-Urethroscopes 8650 E-line design CYSTOSCOPES The E-line design guarantees optimum handling and safe, fatigue-free operation as well as a wide range of possible combinations. Basic Set for Cystoscopy Cysto-urethroscope sheath, 19.5 Fr. with obturator 8650.0341 Adapter with 1 instrument port 8650.264 Insert with Albarran deflector, 2 instrument ports 8650.204 Sterile universal sealing valve (pack of 5) 4712348 Viewing obturator 8650.724 Biopsy forceps Marburg 8650.614 Grasping forceps 8650.684 PANOVIEW telescope, 0° 8650.414 PANOVIEW telescope, 70° 8650.415 Otis urethrotome 8517.00 822.31 822.13 822.31 Flexible connector 822.13 Tray 8585030 1777- 02.01-1118USA 2 PANOVIEW Telescopes Overview CYSTOSCOPES Ø Viewing direction Color code Application mm 0° blue Standard 4 8650.414 12° orange Standard 4 8654.431 Standard 4 8654.422 30° red Long sheath 4 8668.433* 70° yellow Standard 4 8650.415 * Only 25° available. 1777- 02.01-1118USA 3 Cysto-Urethroscope 8650 for telescope 4 mm, 0°, 12°, 30°, 70° and adapters CYSTOSCOPES Sheaths and obturators Adapters Sheath incl. -
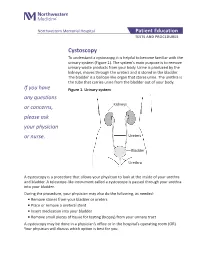
Cystoscopy to Understand a Cystoscopy, It Is Helpful to Become Familiar with the Urinary System (Figure 1)
Northwestern Memorial Hospital Patient Education TESTS AND PROCEDURES Cystoscopy To understand a cystoscopy, it is helpful to become familiar with the urinary system (Figure 1). The system’s main purpose is to remove urinary waste products from your body. Urine is produced by the kidneys, moves through the ureters and is stored in the bladder. The bladder is a balloon-like organ that stores urine. The urethra is the tube that carries urine from the bladder out of your body. If you have Figure 1. Urinary system any questions or concerns, Kidneys please ask your physician or nurse. Ureters Bladder Urethra A cystoscopy is a procedure that allows your physician to look at the inside of your urethra and bladder. A telescope-like instrument called a cystoscope is passed through your urethra into your bladder. During the procedure, your physician may also do the following, as needed: ■ Remove stones from your bladder or ureters ■ Place or remove a ureteral stent ■ Insert medication into your bladder ■ Remove small pieces of tissue for testing (biopsy) from your urinary tract A cystoscopy may be done in a physician’s office or in the hospital’s operating room (OR). Your physician will discuss which option is best for you. Preparation and procedure If the test is done in the OR, you will be asked to sign a written consent. The OR procedure and any special preparation will be explained to you. There may be some discomfort during the examination. Some patients may require sedation or anesthesia. Depending on the type of medication used for your procedure, you will be told if you need to stop eating and drinking before your procedure. -

Urology Services in the ASC
Urology Services in the ASC Brad D. Lerner, MD, FACS, CASC Medical Director Summit ASC President of Chesapeake Urology Associates Chief of Urology Union Memorial Hospital Urologic Consultant NFL Baltimore Ravens Learning Objectives: Describe the numerous basic and advanced urology cases/lines of service that can be provided in an ASC setting Discuss various opportunities regarding clinical, operational and financial aspects of urology lines of service in an ASC setting Why Offer Urology Services in Your ASC? Majority of urologic surgical services are already outpatient Many urologic procedures are high volume, short duration and low cost Increasing emphasis on movement of site of service for surgical cases from hospitals and insurance carriers to ASCs There are still some case types where patients are traditionally admitted or placed in extended recovery status that can be converted to strictly outpatient status and would be suitable for an ASC Potential core of fee-for-service case types (microsurgery, aesthetics, prosthetics, etc.) Increasing Population of Those Aged 65 and Over As of 2018, it was estimated that there were 51 million persons aged 65 and over (15.63% of total population) By 2030, it is expected that there will be 72.1 million persons aged 65 and over National ASC Statistics - 2017 Urology cases represented 6% of total case mix for ASCs Urology cases were 4th in median net revenue per case (approximately $2,400) – behind Orthopedics, ENT and Podiatry Urology comprised 3% of single specialty ASCs (5th behind -

Clinical Significance of Cystoscopic Urethral Stricture
UCSF UC San Francisco Previously Published Works Title Clinical significance of cystoscopic urethral stricture recurrence after anterior urethroplasty: a multi-institution analysis from Trauma and Urologic Reconstructive Network of Surgeons (TURNS). Permalink https://escholarship.org/uc/item/3f57n621 Journal World journal of urology, 37(12) ISSN 0724-4983 Authors Baradaran, Nima Fergus, Kirkpatrick B Moses, Rachel A et al. Publication Date 2019-12-01 DOI 10.1007/s00345-019-02653-6 Peer reviewed eScholarship.org Powered by the California Digital Library University of California World Journal of Urology https://doi.org/10.1007/s00345-019-02653-6 ORIGINAL ARTICLE Clinical signifcance of cystoscopic urethral stricture recurrence after anterior urethroplasty: a multi‑institution analysis from Trauma and Urologic Reconstructive Network of Surgeons (TURNS) Nima Baradaran1 · Kirkpatrick B. Fergus2 · Rachel A. Moses3 · Darshan P. Patel3 · Thomas W. Gaither2 · Bryan B. Voelzke4 · Thomas G. Smith III5 · Bradley A. Erickson6 · Sean P. Elliott7 · Nejd F. Alsikaf8 · Alex J. Vanni9 · Jill Buckley10 · Lee C. Zhao11 · Jeremy B. Myers3 · Benjamin N. Breyer2 Received: 13 December 2018 / Accepted: 24 January 2019 © Springer-Verlag GmbH Germany, part of Springer Nature 2019 Abstract Purpose To assess the functional Queryoutcome of patients with cystoscopic recurrence of stricture post-urethroplasty and to evaluate the role of cystoscopy as initial screening tool to predict future failure. Methods Cases with cystoscopy data after anterior urethroplasty in a multi-institutional database were retrospectively studied. Based on cystoscopic evaluation, performed within 3-months post-urethroplasty, patients were categorized as small-caliber (SC) stricture recurrence: stricture unable to be passed by standard cystoscope, large-caliber (LC) stricture accommodating a cystoscope, and no recurrence. -
Cystoscopy Into the Bladder Via the Urethra, It Is About As Thick As a a Guide for Women
A flexible cystoscope is a thin telescope which is passed Cystoscopy into the bladder via the urethra, it is about as thick as a A Guide for Women pencil. As the cystoscope is flexible, it usually passes easily 1. What is a Cystoscopy? moved so the doctor can look at all the inside lining of the along the curves of the urethra. The flexible tip can also be 2. Why is a Cystoscopy performed? bladder and the opening of the ureters. 3. Preparation for the test 4. About the test 5. Are there any risks? A rigid cystoscope is a shorter rigid telescope, it allows 6. What to expect afterwards? a greater variety of devices to pass down side channels so that the doctor can for example take samples or inject into the bladder. Sometimes, it is necessary to perform a rigid What is a Cystoscopy? Cystoscopy is the name for a procedure allowing a doctor to look into your bladder and urethra with a special telescope cystoscopy at a later date after a flexible cystoscopy. called a cystoscope. Preparation for the test If you are having an outpatient procedure in most cases you When you have a bladder problem, your doctor may use a will be able to eat and drink normally prior to the test. If you cystoscope to see inside your bladder and urethra. The ure- are having general anesthesia, you should refrain from eat- thra is the tube that carries urine from the bladder to the ing and drinking for 6 to 8 hours prior to your cystoscopy. -

Jared J. Wallen MD MBA
Jared J. Wallen MD MBA Education Post American Board of Urology – Board Certification (Expires 02/28/2029) Massachusetts Institute of Technology – Certificate Commercial Real Estate Analysis and Investing (Class of 2019) London School of Economics and Political Science – Certificate Masters of Business Administration Essentials (Class of 2019) Masters in Real Estate Fortune Builders (Class of 2020) Florida Real Estate Sales Associate License Certification (09/2020) Designation as National Commercial Real Estate Advisor and Certified Real Estate Investment Planning Specialist (01/2021) Fell. Boston Scientific Men’s Sexual Health Research Fellow, Class of 2018 P.G. University of Southern Florida – Morsani College of Medicine, Urologic Surgery Residency, Class of 2017 University of Southern Florida – Morsani College of Medicine, General Surgery Internship, Class of 2013 M.D. Rush Medical College, Doctorate in Medicine, Class of 2012 B.S. University of Illinois Urbana/Champaign, Bachelors with Honors in Molecular and Cellular Biology and Psychology, Class of 2008 A.A. Rock Valley Community College, Associates in Arts, Concentration in Psychology, Class of 2004 Experience • Manager and Lead Urologic Surgeon – YOU & WEE Urologic 10/2020 – present Surgery and Wellness • Manager – RUFF & RESTORE EcoFriendly Properties 11/2019 – present • Founder Men’s Health Survivorship Foundation 10/2018 - present • Board Certified Urologic Surgeon – Advent Health Tampa 10/2017 - 10/2020 Physician Group Urology • Adjunct Faculty - Lincoln Memorial University DCOM 02/2018 – 10/2020 • Consultant Boston Scientific 05/2019 – present 05/2019 - present • Consultant Myriad Genetics • Aquatics Student Coordinator Campus Recreation at University 06/2006 - 06/2008 of Illinois • Assistant Store Manager Valvoline Instant Oil Change 06/2001 - 08/2004 Jared J. -

Counter Incision Is a Safe and Effective Method for Alternative Reservoir Placement During Inflatable Penile Prosthesis Surgery
2696 Original Article Counter incision is a safe and effective method for alternative reservoir placement during inflatable penile prosthesis surgery Dominic Grimberg1^, Sabrina Wang2, Evan Carlos1, Brent Nosé1, Shelby Harper2, Aaron C. Lentz1 1Division of Urology, Duke University Medical Center, Durham, NC, USA; 2Duke University School of Medicine, Durham NC, USA Contributions: (I) Conception and Design: D Grimberg, E Carlos, AC Lentz; (II) Administrative support: D Grimberg, B Nosé, S Wang, S Harper; (III) Provision of study materials or patients: AC Lentz; (IV) Collection and assembly of data: D Grimberg, B Nosé, S Wang, S Harper, E Carlos; (V) Data analysis and interpretation: D Grimberg, E Carlos, B Nosé, AC Lentz; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors. Correspondence to: Dr. Dominic Grimberg. Division of Urology, Duke University Medical Center, Room 1573, White Zone, Durham, NC, 27710, USA. Email: [email protected]. Background: Alternative reservoir placement is increasingly popular during inflatable penile prosthesis (IPP) surgery to prevent intraperitoneal positioning, bowel, bladder, or vascular injury in patients with prior pelvic surgeries. Counter incision (CI) can be used for submuscular reservoir placement in high risk patients, however series exploring the safety remain limited. Methods: A database of IPP surgeries was queried for use of a CI during reservoir placement to compare 90-day clinical outcomes in a retrospective case-control study. Primary outcome was device infections, with secondary outcomes including reservoir herniation, hematoma, device malfunction rates, and operative times. Groups were compared using Kruskal-Wallis and Chi-Squared tests, with multivariate logistic regression models to identify predictors of infectious complications. -

Penile Prosthesis Implantation Fried Symposium 2016
Penile Prosthesis Implantation Fried Symposium 2016 Culley C. Carson III, MD, FACS, FRCS(hon) University of North Carolina Chapel Hill, N.C. Management of ED All Patients Sexual/ Physical Exam Laboratory Tests Medical History Discuss Options Noninvasive Tx Additional Tests (Oral, VED, MUSE, PEP) (NPT, Doppler, DICC) Surgical/Invasive Tx ** Penile Prosthesis History • Bogaras(1936)-Rib • Pearman(1967)- cartilage Trimable silicone • Frumkin(1943)-Rib implant cartilage • Morales(1973)- • Scardino (1950)- Intracavernosal Acrylic stent implant • Goodwin & • Small & Scott(1952)-Acrylic Carrion(1973)- stent Intracavernosal • Lash(1964)-Silicone implant implant • Scott(1973)-Inflatable implant Types of Prostheses • Malleable/semirigid – AMS, Coloplast, Jonas • Mechanical rod – AMS Dura II • Inflatable – 2-piece – AMS Ambicor – 3-piece – • AMS 700 (CX, CXR, LGX) • Coloplast (Alpha-1, Titan, Narrow Base) Penile Prosthesis Modifications • Single tubing connectors • Lock-out valve in fluid reservoir or pump to prevent autoinflation • Parylene Coating • Pump Improvements – MS (AMS) – OTR (Coloplast) • Prevention of infections – Antibiotic-impregnated coating (InhibiZone™, AMS) – Hydrophilic/anti-adherence surface (Titan, Mentor) Penile Implant Indications • Oral drug (PDE5 inhibitor) failure • Radical Prostatectomy • Diabetes mellitus • Cardiovascular • Scarred penis – Priapism – Previous implant – Trauma • Peyronie’s disease • Severe venous leak Issues Regarding Informed Consent • Size of penis—usually slight loss in penile length • Possible need -

Diagnosis and Treatment of Neurotrophic Keratopathy
An Evidence-based Approach to the Diagnosis and Treatment of Neurotrophic Keratopathy ACTIVITY DIRECTOR A CME MONOGRAPH Esen K. Akpek, MD This monograph was published by Johns Hopkins School of Medicine in partnership Wilmer Eye Institute with Catalyst Medical Education, LLC. It is Johns Hopkins School of Medicine not affiliated with JAMA medical research Baltimore, Maryland publishing. Visit catalystmeded.com/NK for online testing to earn your CME credit. FACULTY Natalie Afshari, MD Mina Massaro-Giordano, MD Shiley Eye Institute University of Pennsylvania School of Medicine University of California, San Diego Philadelphia, Pennsylvania La Jolla, California Nakul Shekhawat, MD, MPH Sumayya Ahmad, MD Wilmer Eye Institute Mount Sinai School of Medicine Johns Hopkins School of Medicine New York, New York Baltimore, Maryland Pedram Hamrah, MD, FRCS, FARVO Christopher E. Starr, MD Tufts University School of Medicine Weill Cornell Medical College Boston, Massachusetts New York, New York ACTIVITY DIRECTOR FACULTY Esen K. Akpek, MD Natalie Afshari, MD Mina Massaro-Giordano, MD Professor of Ophthalmology Professor of Ophthalmology Professor of Clinical Ophthalmology Director, Ocular Surface Diseases Chief of Cornea and Refractive Surgery University of Pennsylvania School and Dry Eye Clinic Vice Chair of Education of Medicine Wilmer Eye Institute Fellowship Program Director of Cornea Philadelphia, Pennsylvania Johns Hopkins School of Medicine and Refractive Surgery Baltimore, Maryland Shiley Eye Institute Nakul Shekhawat, MD, MPH University of California, -

Selected Non-Moderated Posters
International Journal of Impotence Research (2001) 13, Suppl 2, S17±S21 ß 2001 Nature Publishing Group All rights reserved 0955-9930/01 $15.00 www.nature.com/ijir Selected Non-moderated Posters 12 \' ASECOMY llEHA \'!ORAL EFFECTS IN MEN Authors: T. Bandel; R. Bauer; T. Sigerson. Bayer AG., Wuppertal, Germany Aragao, A J, Rodrigues A 0., Agostinho AG., Juliano, RV. \1arinc!li CM., Affiliation: Pharmacology, Bayer AG, Wuppertal, Germany Wroclavski, ER., Bozzo, I.S Objective: Evaluate the satisfaction degree and the behavioral effecb or vasectomy in the couple and in the man in matter VARDENAFIL'S IMPROVEMENT ON THE QUALITY OF ERECTIONS CAN BE Method There were selected 113 men submit1ed to sterilization by vasectomy, in DEMONSTRATED EARLY IN RIGfSCAN™ STUDIES. the period from January of 1996 to January of 1999, after approval of the Ethics ·commission of Family Planning Service from the Faculdadc de Medicina do ABC. A.JI the patients were called b~· Introduction and Objectives letter. telegram or phone calls, in a spontaneous way and not obligatory A di red questionnaire Vardenafil is a new highly selective PDE5 inhibitor that is being developed for treatment of \Vas used approaching the following: self-esteem aspects, orgasm corHroL sexual satisfaction, erectile dysfunction. ln two Phase Ila studies with patients with mild to moderate erectile complications, sexual habit and masturbation dysfunction, Rigiscan™ measurements of quality of erections were determined. The objective Results: 23,5% of the patients told that, the sexual relationship performance got of this retrospective analysis was to compare the effect ofvardcnafil on the strength of better and 71% didn't notice difference, 5,9% thought worst. -

Endoscopic Vitreoretinal Surgery: Principles, Applications and New Directions Radwan S
Ajlan et al. Int J Retin Vitr (2019) 5:15 International Journal https://doi.org/10.1186/s40942-019-0165-z of Retina and Vitreous REVIEW Open Access Endoscopic vitreoretinal surgery: principles, applications and new directions Radwan S. Ajlan1*, Aarsh A. Desai2 and Martin A. Mainster1 Abstract Purpose: To analyze endoscopic vitreoretinal surgery principles, applications, challenges and potential technological advances. Background: Microendoscopic imaging permits vitreoretinal surgery for tissues that are not visible using operat- ing microscopy ophthalmoscopy. Evolving instrumentation may overcome some limitations of current endoscopic technology. Analysis: Transfer of the fine detail in endoscopic vitreoretinal images to extraocular video cameras is constrained currently by the caliber limitations of intraocular probes in ophthalmic surgery. Gradient index and Hopkins rod lenses provide high resolution ophthalmoscopy but restrict surgical manipulation. Fiberoptic coherent image guides offer surgical maneuverability but reduce imaging resolution. Coaxial endoscopic illumination can highlight delicate vitreo- retinal structures difficult to image in chandelier or endoilluminator diffuse, side-scattered lighting. Microendoscopy’s ultra-high magnification video monitor images can reveal microscopic tissue details blurred partly by ocular media aberrations in contemporary surgical microscope ophthalmoscopy, thereby providing a lower resolution, invasive alternative to confocal fundus imaging. Endoscopic surgery is particularly useful when ocular -

Visual Outcome of Pars Plana Vitrectomy Following Endophthalmitis – a Vitreo-Retinal Surgeon's Experience
Medical Journal of Zambia, Vol. 47 (1): 33 - 38 (2020) Original Article Visual outcome of Pars Plana Vitrectomy following Endophthalmitis – A Vitreo-retinal Surgeon's Experience Sanjoy K. Das1 ,Osayem J. Otabor-Olubor2, Situma P Wanyama1 1Ispahani Islamia Eye Institute and Hospital (IIEIH), Dhaka, Bangladesh 2Ispahani Islamia Eye Institute and Hospital (IIEIH), Dhaka, Bangladesh1 and University of Benin Teaching Hospital (UBTH), Benin City, Nigeria. ABSTRACT (p-value = 0.007). There was an association (although not strong) between age range and post- Purpose: To determine the visual outcome of pars operative visual improvement (p-value = 0.639). plana vitrectomy following endophthalmitis. Conclusion: Pars plana vitrectomy helps to prevent Methods: A retrospective hospital-based study of 20 or minimize ophthalmic complications from eyes of consecutive patients who had pars plana endophthalmitis, thus making it a necessary line of vitrectomy by a particular surgeon following treatment endophthalmitis. Pars plana vitrectomy is endophthalmitis in the Ispahani Islamia Eye therefore a safe and desirable definitive treatment Institute and Hospital, Dhaka, Bangladesh from for endophthalmitis, and it confers a good chance of August – December 2017. Using the Snellen's visual visual improvement. acuity chart, post-operative distant visual acuity improvement was graded 0– 9, with 0 meaning no INTRODUCTION improvement in visual acuity, and 9 meaning nine Pars plana vitrectomy (the intricate surgical removal lines of improvement in visual acuity from of vitreous gel from the vitreous cavity) has seen presenting best corrected visual acuity. remarkable progress in surgical technique and post- Results: Pre-operative corrected distant visual operative outcome in ophthalmic practices all over acuity (CDVA) ranged from perception of light to the world.