Soft-Tissue Anatomy of the Extant Hominoids Anatomy of the Extant Hominoids: a Review and Phylogenetic Analysis S
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
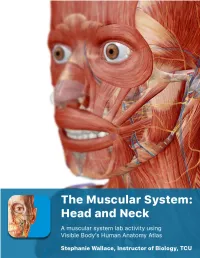
The Muscular System Views
1 PRE-LAB EXERCISES Before coming to lab, get familiar with a few muscle groups we’ll be exploring during lab. Using Visible Body’s Human Anatomy Atlas, go to the Views section. Under Systems, scroll down to the Muscular System views. Select the view Expression and find the following muscles. When you select a muscle, note the book icon in the content box. Selecting this icon allows you to read the muscle’s definition. 1. Occipitofrontalis (epicranius) 2. Orbicularis oculi 3. Orbicularis oris 4. Nasalis 5. Zygomaticus major Return to Muscular System views, select the view Head Rotation and find the following muscles. 1. Sternocleidomastoid 2. Scalene group (anterior, middle, posterior) 2 IN-LAB EXERCISES Use the following modules to guide your exploration of the head and neck region of the muscular system. As you explore the modules, locate the muscles on any charts, models, or specimen available. Please note that these muscles act on the head and neck – those that are located in the neck but act on the back are in a separate section. When reviewing the action of a muscle, it will be helpful to think about where the muscle is located and where the insertion is. Muscle physiology requires that a muscle will “pull” instead of “push” during contraction, and the insertion is the part that will move. Imagine that the muscle is “pulling” on the bone or tissue it is attached to at the insertion. Access 3D views and animated muscle actions in Visible Body’s Human Anatomy Atlas, which will be especially helpful to visualize muscle actions. -

Male Reproductive System 2
Male Reproductive System 2 1. Excretory genital ducts 2. The ductus (vas) deferens and seminal vesicles 3. The prostate 4. The bulbourethral (Cowper’s) glands 5. The penis 6. The scrotum and spermatic cord SPLANCHNOLOGY Male reproductive system ° Male reproductive system, systema genitalia masculina: V a part of the human reproductive process ° Male reproductive organs, organa genitalia masculina: V internal genital organs: testicle, testis epididymis, epididymis ductus deferens, ductus (vas) deferens seminal vesicle, vesicula seminalis ejaculatory duct, ductus ejaculatorius prostate gland, prostata V external genital organs: penis, penis scrotum, scrotum bulbourethral glands, glandulae bulbourethrales Prof. Dr. Nikolai Lazarov 2 SPLANCHNOLOGY Ductus (vas) deferens ° Ductus (vas) deferens: V a straight thick-walled muscular tube V transports sperm cells from the epididymis V length 45-50 cm V diameter 2.5-3 mm ° Anatomical parts: V testicular part V funicular part V inguinal part – 4 cm V pelvic part ° Ampulla ductus deferentis: V length 3-4 cm; diameter 1 cm V ejaculatory duct, ductus ejaculatorius Prof. Dr. Nikolai Lazarov 3 SPLANCHNOLOGY Microscopic anatomy ° tunica mucosa – 5-6 longitudinal folds: V lamina epithelialis – bilayered columnar epithelium with stereocilia V lamina propria: dense connective tissue elastic fibers ° tunica muscularis – thick: V inner longitudinal layer – in the initial portion V circular layer V outer longitudinal layer ° tunica adventitia (serosa) Prof. Dr. Nikolai Lazarov 4 SPLANCHNOLOGY Seminal vesicle, vesicula seminalis ° Seminal vesicle, vesicula (glandula) seminalis: V a pair of simple tubular glands – two highly tortuous tubes V posterior to the urinary bladder V length 4-5 (15) cm V diameter 1 cm ° Macroscopic anatomy: V anterior and posterior part V excretory duct Prof. -

Axis Scientific Skull with Muscle Origins and Insertions A-108851
Axis Scientific Skull with Muscle Origins and Insertions A-108851 *Muscle Origins = RED Anterior View Occipital Bone Posterior View *Muscle Insertions = BLUE Posterior Cerebral Artery Frontal Bone Basilar Artery Pontine Arteries Parietal Bone Nasal Bone Temporal Bone Sphenoid Bone Temporalis Parietal Bone Occipitofrontalis Occipital Bone Lacrimal Bone Sternocleidomastoid Trapezius Temporalis Semispinalis Capitis Temporal Bone Corrugator Supercilii Splenius Capitis Longissimus Capitis Orbicularis Oculi Obliquus Capitis Superior Temporal Basilar Artery Bone Procerus Rectis Capitis C1 Posterior Major Vertebral Artery Levator Labii Superioris C2 Alaeque Nasi Rectis Capitis Posterior Minor Sphenoid Levator Labii Superioris Posterior Digastric Zygomatic Bone Bone C3 Nasalis (Transverse Part) Rectis Capitis C4 Masseter Lateralis Spinal Nerve Zygomaticus Major C5 Zygomatic Medial Pterygoid Bone Zygomaticus Minor C6 Temporalis Mylohyoid C7 Mandible Levator Anguli Oris Vomer Nasalis (Alar Part) Spinal Nerve Spinal Cord Maxilla Orbicularis Oris Depressor Septi Nasi Masseter Medial Superior Masseter Mandible Orbicularis Oris Constrictor Pterygoid Inferior View Styloglossus Platysma Mylohyoid Depressor Stylohyoid Anguli Oris Anterior Stylopharyngeus Spinal Nerve Depressor Digastric Labii Inferioris Posterior Geniohyoid Digastric Vertebral Artery Mentalis Rectis Capitis Genioglossus Lateralis Rectis Capitis Buccinator Mandible Posterior Major Rectis Capitis Posterior Minor Frontal Bone Corrugator Supercilii Orbicularis Oculi Lacrimal Bone Depressor -

Questions on Human Anatomy
Standard Medical Text-books. ROBERTS’ PRACTICE OF MEDICINE. The Theory and Practice of Medicine. By Frederick T. Roberts, m.d. Third edi- tion. Octavo. Price, cloth, $6.00; leather, $7.00 Recommended at University of Pennsylvania. Long Island College Hospital, Yale and Harvard Colleges, Bishop’s College, Montreal; Uni- versity of Michigan, and over twenty other medical schools. MEIGS & PEPPER ON CHILDREN. A Practical Treatise on Diseases of Children. By J. Forsyth Meigs, m.d., and William Pepper, m.d. 7th edition. 8vo. Price, cloth, $6.00; leather, $7.00 Recommended at thirty-five of the principal medical colleges in the United States, including Bellevue Hospital, New York, University of Pennsylvania, and Long Island College Hospital. BIDDLE’S MATERIA MEDICA. Materia Medica, for the Use of Students and Physicians. By the late Prof. John B Biddle, m.d., Professor of Materia Medica in Jefferson Medical College, Phila- delphia. The Eighth edition. Octavo. Price, cloth, $4.00 Recommended in colleges in all parts of the UnitedStates. BYFORD ON WOMEN. The Diseases and Accidents Incident to Women. By Wm. H. Byford, m.d., Professor of Obstetrics and Diseases of Women and Children in the Chicago Medical College. Third edition, revised. 164 illus. Price, cloth, $5.00; leather, $6.00 “ Being particularly of use where questions of etiology and general treatment are concerned.”—American Journal of Obstetrics. CAZEAUX’S GREAT WORK ON OBSTETRICS. A practical Text-book on Midwifery. The most complete book now before the profession. Sixth edition, illus. Price, cloth, $6.00 ; leather, $7.00 Recommended at nearly fifty medical schools in the United States. -

The Articulatory System Chapter 6 Speech Science/ COMD 6305 UTD/ Callier Center William F. Katz, Ph.D
The articulatory system Chapter 6 Speech Science/ COMD 6305 UTD/ Callier Center William F. Katz, Ph.D. STRUCTURE/FUNCTION VOCAL TRACT CLASSIFICATION OF CONSONANTS AND VOWELS MORE ON RESONANCE ACOUSTIC ANALYSIS/ SPECTROGRAMS SUPRSEGMENTALS, COARTICULATION 1 Midsagittal dissection From Kent, 1997 2 Oral Cavity 3 Oral Structures – continued • Moistened by saliva • Lined by mucosa • Saliva affected by meds 4 Tonsils • PALATINE* (laterally – seen in oral periph • LINGUAL (inf.- root of tongue) • ADENOIDS (sup.) [= pharyngeal] • Palatine, lingual tonsils are larger in children • *removed in tonsillectomy 5 Adenoid Facies • Enlargement from infection may cause problems (adenoid facies) • Can cause problems with nasal sounds or voicing • Adenoidectomy; also tonsillectomy (for palatine tonsils) 6 Adenoid faces (example) 7 Oral structures - frenulum Important component of oral periphery exam Lingual frenomy – for ankyloglossia “tongue-tie” Some doctors will snip for infants, but often will loosen by itself 8 Hard Palate Much variability in palate shape and height Very high vault 9 Teeth 10 Dentition - details Primary (deciduous, milk teeth) Secondary (permanent) n=20: n=32: ◦ 2 incisor ◦ 4 incisor ◦ 1 canine ◦ 2 canine ◦ 2 molar ◦ 4 premolar (bicuspid) Just for “fun” – baby ◦ 6 molar teeth pushing in! NOTE: x 2 for upper and lower 11 Types of malocclusion • Angle’s classification: • I, II, III • Also, individual teeth can be misaligned (e.g. labioversion) Also “Neutrocclusion/ distocclusion/mesiocclusion” 12 Dental Occlusion –continued Other terminology 13 Mandible Action • Primary movements are elevation and depression • Also…. protrusion/retraction • Lateral grinding motion 14 Muscles of Jaw Elevation Like alligators, we are much stronger at jaw elevation (closing to head) than depression 15 Jaw Muscles ELEVATORS DEPRESSORS •Temporalis ✓ •Mylohyoid ✓ •Masseter ✓ •Geniohyoid✓ •Internal (medial) Pterygoid ✓ •Anterior belly of the digastric (- Kent) •Masseter and IP part of “mandibular sling” •External (lateral) pterygoid(?)-- also protrudes and rocks side to side. -

Spermatogonia, Primary and Secondary Spermatocytes, and Early and Late Spermatids
David (Michelangelo) MED316 REPRODUCTIVE SYSTEM AND DISORDERS HISTOLOGY OF THE MALE REPRODUCTIVE SYSTEM Histology of Testes and Spermatogenesis Dr. Sinan Özkavukcu Ankara University Faculty of Medicine Dept. of Histology – Embryology Lab Director - Center for Assisted Reproduction Male Reproductive System • Testes • Genital excurrent ducts • Ductuli efferentes • Ductus epididymis • Ductus deferens • Accessory sex glands • Seminal vesicles • Prostate • Bulbourethral glands • External genitalia • Penis • Scrotum Endocrine control of male reproduction • Onset of puberty • brain determines the timing of onset of puberty • crucial role in controlling sexual behavior and reproduction is played by the hypothalamus • Terminals of gonadotropin-releasing hormone (GnRH)-secreting neurons release their secretions in the median eminence and infundibulum, where they enter the hypophyseal portal system • GnRH is then driven to the anterior pituitary, precisely to the gonadotrophs, basophil-staining cells, which constitute 10%– 15% of anterior pituitary cells and are located throughout the entire anterior lobe • The gonadotrophs synthesize follicle-stimulating hormone (FSH) and luteinizing hormone (LH) and release them into the systemic circulation; both hormones reach the testis by testicular arteries. An Introduction to Male Reproductive Medicine, Niederberger Anti-Müllerian Hormone in Disorders of Sex Determination and Differentiation Arq Bras Endocrinol Metab vol 49 nº 1 Fevereiro 2005 Circulation of testes • The arterial supply to the testes follows the lobular division of seminiferous tubules, • Each lobulus is supplied by one recurrent artery; segmental arteries and capillaries become branched between the Leydig cells and then give rise to the venous system. • The pattern of blood supply to the testis is also essential for maintaining a lower testicular temperature compared with body temperature. -

Pdf Manual (964.7Kb)
MD-17 , CONTENTS THE URINARY SYSTEM 4 THE REPRODUCTIVE SYSTEM 5 The Scrotum The Testis The Epididylnis The Ductus Deferens The Ejaculatory Duct The Seminal Vesicle The Spermatic Cord The Penis The Prostate Gland THE INGUINAL CANAL l) HERNIAS FURTIlER READING 10 MODEL KEY 1I Human Male Pelvis This life-size model shows the viscera and structures which form the urogenital system and some of the related anatomy such as the sig moid colon and rectum. The vascular supply to the viscera and support ing tissue is demonstrated, as well as that portion of the vascular system which continues into the lower extremity. The model is divided into right and left portions. The right portion shows a midsagittal section of the pelvic structures. The left represents a similar section, but the dissection is deeper. Two pieces are remov able on the left side; one piece includes the bladder, prostate, and semi nal vesicles, and the other includes the penis, left testicle, and scrotum. When all portions are removed, a deeper view of these structures and a deeper dissection of the pelvis can be seen. THE URINARY SYSTEM The portion of the urinary system shown depicts the ureter from the level of the 5th lumbar vertebra, where it passes the common iliac ar tery near the bifurcation of thi s artery into the external and internal iliac arteries. The ureter then passes toward the posterior portion of the bladder, beneath the vas deferens, and opens through the wall of the blad der at one cranial corner of the trigone on the bladder's interior. -

Methodical Complex on Gross Anatomy for Ii Course
MINISTRY OF HIGHER AND SECONDARY SPECIAL EDUCATION OF UZBEKISTAN BUKHARA STATE MEDICAL INSTITUTE NAMED AFTER ABU ALI IBN SINO DEPARTMENT OF ANATOMY "APPROVED" by Vice-Rector for Academic and educational work, Associate prof. G.J.Jarilkasinova ________________________________ "_____" ________________ 2020 Area of knowledge: 500000 - Health and social care Education field: 510000 - Healthcare Educational direction: 5510100 - Medical business 5111000 - Professional education (5510100 - Medicine business) 5510200 - Pediatric Medicine 5510300 - Medico-prophylactic business 5510400 – Dentistry (by directions) 5510900 – Medico-biological business EDUCATIONAL - METHODICAL COMPLEX ON GROSS ANATOMY FOR II COURSE Bukhara 2020 The scientific program was approved by the Resolution of the Coordination Council No. ___ of August ___, 2020 on the activities of educational and methodological associations in the areas of higher and secondary special and vocational education. The teaching and methodical complex was developed by order of the Ministry of Higher and Secondary Special Education of the Republic of Uzbekistan dated March 1, 2017 No. 107. Compilers: Radjabov A.B. - Head of the Department of Anatomy, Associate Professor Khasanova D.A. - Assistant of the Department of Anatomy, PhD Bobomurodov N.L. - Associate Professor of the Department of Anatomy Reviewers: Davronov R.D. - Head of the Department Histology and Medical biology, Associate Professor Djuraeva G.B. - Head of the Department of the Department of Pathological Anatomy and Judicial Medicine, Associate Professor The working educational program for anatomy is compiled on the basis of working educational curriculum and educational program for the areas of 5510100 - Medical business. This is discussed and approved at the department Protocol № ______ of "____" _______________2020 Head of the chair, associate professor: Radjabov A.B. -

The Myloglossus in a Human Cadaver Study: Common Or Uncommon Anatomical Structure? B
Folia Morphol. Vol. 76, No. 1, pp. 74–81 DOI: 10.5603/FM.a2016.0044 O R I G I N A L A R T I C L E Copyright © 2017 Via Medica ISSN 0015–5659 www.fm.viamedica.pl The myloglossus in a human cadaver study: common or uncommon anatomical structure? B. Buffoli*, M. Ferrari*, F. Belotti, D. Lancini, M.A. Cocchi, M. Labanca, M. Tschabitscher, R. Rezzani, L.F. Rodella Section of Anatomy and Physiopathology, Department of Clinical and Experimental Sciences, University of Brescia, Brescia, Italy [Received: 1 June 2016; Accepted: 18 July 2016] Background: Additional extrinsic muscles of the tongue are reported in literature and one of them is the myloglossus muscle (MGM). Since MGM is nowadays considered as anatomical variant, the aim of this study is to clarify some open questions by evaluating and describing the myloglossal anatomy (including both MGM and its ligamentous counterpart) during human cadaver dissections. Materials and methods: Twenty-one regions (including masticator space, sublin- gual space and adjacent areas) were dissected and the presence and appearance of myloglossus were considered, together with its proximal and distal insertions, vascularisation and innervation. Results: The myloglossus was present in 61.9% of cases with muscular, ligamen- tous or mixed appearance and either bony or muscular insertion. Facial artery pro- vided myloglossal vascularisation in the 84.62% and lingual artery in the 15.38%; innervation was granted by the trigeminal system (buccal nerve and mylohyoid nerve), sometimes (46.15%) with hypoglossal component. Conclusions: These data suggest us to not consider myloglossus as a rare ana- tomical variant. -

Head & Neck Muscle Table
Robert Frysztak, PhD. Structure of the Human Body Loyola University Chicago Stritch School of Medicine HEAD‐NECK MUSCLE TABLE PROXIMAL ATTACHMENT DISTAL ATTACHMENT MUSCLE INNERVATION MAIN ACTIONS BLOOD SUPPLY MUSCLE GROUP (ORIGIN) (INSERTION) Anterior floor of orbit lateral to Oculomotor nerve (CN III), inferior Abducts, elevates, and laterally Inferior oblique Lateral sclera deep to lateral rectus Ophthalmic artery Extra‐ocular nasolacrimal canal division rotates eyeball Inferior aspect of eyeball, posterior to Oculomotor nerve (CN III), inferior Depresses, adducts, and laterally Inferior rectus Common tendinous ring Ophthalmic artery Extra‐ocular corneoscleral junction division rotates eyeball Lateral aspect of eyeball, posterior to Lateral rectus Common tendinous ring Abducent nerve (CN VI) Abducts eyeball Ophthalmic artery Extra‐ocular corneoscleral junction Medial aspect of eyeball, posterior to Oculomotor nerve (CN III), inferior Medial rectus Common tendinous ring Adducts eyeball Ophthalmic artery Extra‐ocular corneoscleral junction division Passes through trochlea, attaches to Body of sphenoid (above optic foramen), Abducts, depresses, and medially Superior oblique superior sclera between superior and Trochlear nerve (CN IV) Ophthalmic artery Extra‐ocular medial to origin of superior rectus rotates eyeball lateral recti Superior aspect of eyeball, posterior to Oculomotor nerve (CN III), superior Elevates, adducts, and medially Superior rectus Common tendinous ring Ophthalmic artery Extra‐ocular the corneoscleral junction division -

Anatomy and Physiology Male Reproductive System References
DEWI PUSPITA ANATOMY AND PHYSIOLOGY MALE REPRODUCTIVE SYSTEM REFERENCES . Tortora and Derrickson, 2006, Principles of Anatomy and Physiology, 11th edition, John Wiley and Sons Inc. Medical Embryology Langeman, pdf. Moore and Persaud, The Developing Human (clinically oriented Embryologi), 8th edition, Saunders, Elsevier, . Van de Graff, Human anatomy, 6th ed, Mcgraw Hill, 2001,pdf . Van de Graff& Rhees,Shaum_s outline of human anatomy and physiology, Mcgraw Hill, 2001, pdf. WHAT IS REPRODUCTION SYSTEM? . Unlike other body systems, the reproductive system is not essential for the survival of the individual; it is, however, required for the survival of the species. The RS does not become functional until it is “turned on” at puberty by the actions of sex hormones sets the reproductive system apart. The male and female reproductive systems complement each other in their common purpose of producing offspring. THE TOPIC : . 1. Gamet Formation . 2. Primary and Secondary sex organ . 3. Male Reproductive system . 4. Female Reproductive system . 5. Female Hormonal Cycle GAMET FORMATION . Gamet or sex cells are the functional reproductive cells . Contain of haploid (23 chromosomes-single) . Fertilizationdiploid (23 paired chromosomes) . One out of the 23 pairs chromosomes is the determine sex sex chromosome X or Y . XXfemale, XYmale Gametogenesis Oocytes Gameto Spermatozoa genesis XY XX XX/XY MALE OR FEMALE....? Male Reproductive system . Introduction to the Male Reproductive System . Scrotum . Testes . Spermatic Ducts, Accessory Reproductive Glands,and the Urethra . Penis . Mechanisms of Erection, Emission, and Ejaculation The urogenital system . Functionally the urogenital system can be divided into two entirely different components: the urinary system and the genital system. -

Making Faces
Making Faces Chris Landreth CSC2529, Session 4 31 January 2011 AU1,2 (Frontalis): 2 AU4 (Corrugator): 1 AU5 (Levitor Palpabrae): 3 AU6,44 (Orbicularis Oculi): 6 How AU9 (Alaeque Nasi Labius Superioris): 1 AU10 (Labius Superioris): 3 many AU12 (Zygomatic Major): 3 letters in AU14 (Buccinator): 3 AU15 (Triangularis): 3 this AU16 (Labius Inferioris): 1 alphabet? AU17 (Mentalis): 1 AU18 (Incisivus): 1 AU20 (Risorius/Platysma): 3 AU22,23 (Orbicularis Oris): 6 AU26 (Jaw): 4 _________________________________________________ TOTAL: 41 AU’s Putting the letters together into words: Expressions The six fundamental expressions: 1. Anger 2. Sadness 3. Disgust 4. Surprise 5. Fear 6. Happiness The six fundamental expressions: 1. Anger 2. Sadness 3. Disgust 4. Surprise 5. Fear 6. Happiness A Few Words of Anger Glaring: A Few Words of Anger Glaring: Slight creases in the middle brow (Currogator) Eyelids are slightly raised (Levitor Palpabrae) Lips are clenched backward (Buccinator) Slight downturn in lip corners (Triangularis) A Few Words of Anger Miffed: A Few Words of Anger Miffed: Classic, angry ‘v-shaped’ eyebrows (Currogator) Nasolabial fold deepens, Upper lip is squared off (A.N. Labius Superioris) Lower lip raises into a pout, Dimpling in the chin (Mentalis) A Few Words of Anger Pissed off: A Few Words of Anger Pissed off: Brow raises slightly (Frontalis) Sharper Nasolabial Fold, Raised upper lip (A.N. Labius Superioris) Lower lip juts out (Orb. Oris, Lower Lip out) A Few Words of Anger Very Pissed off: A Few Words of Anger Very Pissed off: Slight squinting (Orb. Oculi) Bared upper teeth (Orb. Oris, Upper Lip Out) Squared lower lip corners, Sharp tendon creases in her neck (Risorius/Platysma) A Few Words of Anger Consumed in Rage: A Few Words of Anger Consumed in Rage: Intense, asymmetrical squinting (Orb.