5.6 Free-Radical Addition of Hydrogen Bromide to Alkenes 201
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Part I: Carbonyl-Olefin Metathesis of Norbornene
Part I: Carbonyl-Olefin Metathesis of Norbornene Part II: Cyclopropenimine-Catalyzed Asymmetric Michael Reactions Zara Maxine Seibel Submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Graduate School of Arts and Sciences COLUMBIA UNIVERSITY 2016 1 © 2016 Zara Maxine Seibel All Rights Reserved 2 ABSTRACT Part I: Carbonyl-Olefin Metathesis of Norbornene Part II: Cyclopropenimine-Catalyzed Asymmetric Michael Reactions Zara Maxine Seibel This thesis details progress towards the development of an organocatalytic carbonyl- olefin metathesis of norbornene. This transformation has not previously been done catalytically and has not been done in practical manner with stepwise or stoichiometric processes. Building on the previous work of the Lambert lab on the metathesis of cyclopropene and an aldehyde using a hydrazine catalyst, this work discusses efforts to expand to the less stained norbornene. Computational and experimental studies on the catalytic cycle are discussed, including detailed experimental work on how various factors affect the difficult cycloreversion step. The second portion of this thesis details the use of chiral cyclopropenimine bases as catalysts for asymmetric Michael reactions. The Lambert lab has previously developed chiral cyclopropenimine bases for glycine imine nucleophiles. The scope of these catalysts was expanded to include glycine imine derivatives in which the nitrogen atom was replaced with a carbon atom, and to include imines derived from other amino acids. i Table of Contents List of Abbreviations…………………………………………………………………………..iv Part I: Carbonyl-Olefin Metathesis…………………………………………………………… 1 Chapter 1 – Metathesis Reactions of Double Bonds………………………………………….. 1 Introduction………………………………………………………………………………. 1 Olefin Metathesis………………………………………………………………………… 2 Wittig Reaction…………………………………………………………………………... 6 Tebbe Olefination………………………………………………………………………... 9 Carbonyl-Olefin Metathesis……………………………………………………………. -

Title Crystallization of Stereospecific Olefin Copolymers (Special Issue on Physical Chemistry) Author(S) Sakaguchi, Fumio; Kita
Crystallization of Stereospecific Olefin Copolymers (Special Title Issue on Physical Chemistry) Author(s) Sakaguchi, Fumio; Kitamaru, Ryozo; Tsuji, Waichiro Bulletin of the Institute for Chemical Research, Kyoto Citation University (1966), 44(4): 295-315 Issue Date 1966-10-31 URL http://hdl.handle.net/2433/76134 Right Type Departmental Bulletin Paper Textversion publisher Kyoto University Crystallization of Stereospecifie Olefin Copolymers Fumio SAKAGUCHI,Ryozo KITAMARU and Waichiro TSUJI* (Tsuji Laboratory) Received August 13, 1966 The stereoregularity of isotactic poly(4-methyl-1-pentene) was characterized and isomorphism phenomena were examined for the copolymeric systems of 4-methyl-1-pentene with several olefins in order to study the crystallization phenomena in these olefin copoly- mers polymerized with stereospecific catalysts. The structural heterogeneity or the fine crystalline structure of poly(4-methyl-1-pentene) could be correlated with its molecular structure by viewing this stereoregular homopolymer as if it were a copolymer. Cocrystallization or isomorphism phenomenon was recognized for the copolymeric systems of 4-methyl-1-pentene with butene-1, pentene-1, decene-1 and 3-methyl-1-butene, while no evidence of the phenomenon was obtained for the copolymeric systems with styrene and propylene. The degree of the isomorphism of those copolymers was discussed with the informations on the crystalline phases obtained from the X-ray study, on the constitution of the copolymeric chains in the amorphous phases obtained from the viscoelastic studies and on the other thermodynamical properties of these systems. INTRODUCTION Many works have been made with regard to the homopolymerization of olefins with stereospecific catalysts, i. e. complex catalysts composed of the combination of organometallic compound and transitional metallic compound. -

Syntheses and Eliminations of Cyclopentyl Derivatives David John Rausch Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1966 Syntheses and eliminations of cyclopentyl derivatives David John Rausch Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Organic Chemistry Commons Recommended Citation Rausch, David John, "Syntheses and eliminations of cyclopentyl derivatives " (1966). Retrospective Theses and Dissertations. 2875. https://lib.dr.iastate.edu/rtd/2875 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. This dissertation has been microfilmed exactly as received 66—6996 RAUSCH, David John, 1940- SYNTHESES AND ELIMINATIONS OF CYCLOPENTYL DERIVATIVES. Iowa State University of Science and Technology Ph.D., 1966 Chemistry, organic University Microfilms, Inc., Ann Arbor, Michigan SYNTHESES AND ELIMINATIONS OF CYCLOPENTYL DERIVATIVES by David John Rausch A Dissertation Submitted to the Graduate Faculty in Partial Fulfillment of The Requirements for the Degree of DOCTOR OF PHILOSOPHY Major Subject: Organic Chemistry Approved : Signature was redacted for privacy. Signature was redacted for privacy. Head of Major Department Signature was redacted for privacy. Iowa State University Of Science and Technology Ames, Iowa 1966 ii TABLE OF CONTENTS VITA INTRODUCTION HISTORICAL Conformation of Cyclopentanes Elimination Reactions RESULTS AND DISCUSSION Synthetic Elimination Reactions EXPERIMENTAL Preparation and Purification of Materials Procedures and Data for Beta Elimination Reactions SUMMARY LITERATURE CITED ACKNOWLEDGEMENTS iii VITA The author was born in Aurora, Illinois, on October 24, 1940, to Mr. -

Properties and Synthesis. Elimination Reactions of Alkyl Halides
P1: PBU/OVY P2: PBU/OVY QC: PBU/OVY T1: PBU Printer: Bind Rite JWCL234-07 JWCL234-Solomons-v1 December 8, 2009 21:37 7 ALKENES AND ALKYNES I: PROPERTIES AND SYNTHESIS. ELIMINATION REACTIONS OF ALKYL HALIDES SOLUTIONS TO PROBLEMS 7.1 (a) ( E )-1-Bromo-1-chloro-1-pentene or ( E )-1-Bromo-1-chloropent-1-ene (b) ( E )-2-Bromo-1-chloro-1-iodo-1-butene or ( E )-2-Bromo-1-chloro-1-iodobut-1-ene (c) ( Z )-3,5-Dimethyl-2-hexene or ( Z )-3,5-Dimethylhex-2-ene (d) ( Z )-1-Chloro-1-iodo-2-methyl-1-butene or ( Z )-1-Chloro-1-iodo-2-methylbut-1-ene (e) ( Z,4 S )-3,4-Dimethyl-2-hexene or ( Z,4 S )-3,4-Dimethylhex-2-ene (f) ( Z,3 S )-1-Bromo-2-chloro-3-methyl-1-hexene or (Z,3 S )-1-Bromo-2-chloro-3-methylhex-1-ene 7.2 < < Order of increasing stability 7.3 (a), (b) H − 2 ∆ H° = − 119 kJ mol 1 Pt 2-Methyl-1-butene pressure (disubstituted) H2 − ∆ H° = − 127 kJ mol 1 Pt 3-Methyl-1-butene pressure (monosubstituted) H2 − ∆ H° = − 113 kJ mol 1 Pt 2-Methyl-2-butene pressure (trisubstituted) (c) Yes, because hydrogenation converts each alkene into the same product. 106 CONFIRMING PAGES P1: PBU/OVY P2: PBU/OVY QC: PBU/OVY T1: PBU Printer: Bind Rite JWCL234-07 JWCL234-Solomons-v1 December 8, 2009 21:37 ALKENES AND ALKYNES I: PROPERTIES AND SYNTHESIS 107 H H (d) > > H H H (trisubstituted) (disubstituted) (monosubstituted) Notice that this predicted order of stability is confirmed by the heats of hydro- genation. -

Chem 51B Chapter 15 Notes
Lecture Notes Chem 51B S. King Chapter 15 Radical Reactions I. Introduction A radical is a highly reactive intermediate with an unpaired electron. Radicals are involved in oxidation reactions, combustion reactions, and biological reactions. Structure: compare with: compare with: Stability: Free radicals and carbocations are both electron deficient and they follow a similar order of stability: R R H H , > R C > R C > R C > H C R H H H • like carbocations, radicals can be stabilized by resonance. H H H H C C C C H C CH3 H C CH3 H H • unlike carbocations, no rearrangements are observed in free radical reactions. Q. How are free radicals formed? A. Free radicals are formed when bonds break homolytically: 103 Look @ the arrow pushing: Notice the fishhook arrow! It shows movement of a single electron: Compare with heterolytic bond cleavage: A double-headed arrow shows movement of a pair of electrons: Nomenclature: bromide ion bromine atom Bromine (molecule) II. General Features of Radical Reactions Radicals are formed from covalent bonds by adding energy in the form of heat or light (hν). Some radical reactions are carried out in the presence of radical initiators, which contain weak bonds that readily undergo homolysis. The most common radical initiators are peroxides (ROOR), which contain the weak O−O bond. A. Common Reactions of Radicals: Radicals undergo two common reactions: they react with σ-bonds and they add to π-bonds. 1. Reaction of a Radical X• with a C−H bond A radical X• abstracts a H atom from a C−H bond to form H−X and a carbon radical: 104 2. -

Reactions of Alkenes and Alkynes
05 Reactions of Alkenes and Alkynes Polyethylene is the most widely used plastic, making up items such as packing foam, plastic bottles, and plastic utensils (top: © Jon Larson/iStockphoto; middle: GNL Media/Digital Vision/Getty Images, Inc.; bottom: © Lakhesis/iStockphoto). Inset: A model of ethylene. KEY QUESTIONS 5.1 What Are the Characteristic Reactions of Alkenes? 5.8 How Can Alkynes Be Reduced to Alkenes and 5.2 What Is a Reaction Mechanism? Alkanes? 5.3 What Are the Mechanisms of Electrophilic Additions HOW TO to Alkenes? 5.1 How to Draw Mechanisms 5.4 What Are Carbocation Rearrangements? 5.5 What Is Hydroboration–Oxidation of an Alkene? CHEMICAL CONNECTIONS 5.6 How Can an Alkene Be Reduced to an Alkane? 5A Catalytic Cracking and the Importance of Alkenes 5.7 How Can an Acetylide Anion Be Used to Create a New Carbon–Carbon Bond? IN THIS CHAPTER, we begin our systematic study of organic reactions and their mecha- nisms. Reaction mechanisms are step-by-step descriptions of how reactions proceed and are one of the most important unifying concepts in organic chemistry. We use the reactions of alkenes as the vehicle to introduce this concept. 129 130 CHAPTER 5 Reactions of Alkenes and Alkynes 5.1 What Are the Characteristic Reactions of Alkenes? The most characteristic reaction of alkenes is addition to the carbon–carbon double bond in such a way that the pi bond is broken and, in its place, sigma bonds are formed to two new atoms or groups of atoms. Several examples of reactions at the carbon–carbon double bond are shown in Table 5.1, along with the descriptive name(s) associated with each. -

Peroxides and Peroxide- Forming Compounds
FEATURE Peroxides and peroxide- forming compounds By Donald E. Clark Bretherick5 included a discussion of nated. However, concentrated hydro- organic peroxide5 in a chapter on gen peroxide (Ͼ30%), in contact with norganic and organic peroxides, highly reactive and unstable com- ordinary combustible materials (e.g., because of their exceptional reac- pounds and used “oxygen balance” to fabric, oil, wood, or some resins) Itivity and oxidative potential are predict the stability of individual com- poses significant fire or explosion haz- widely used in research laboratories. pounds and to assess the hazard po- ards. Peroxides of alkali metals are not This review is intended to serve as a tential of an oxidative reaction. Jack- particularly shock sensitive, but can 6 guide to the hazards and safety issues son et al. addressed the use of decompose slowly in the presence of associated with the laboratory use, peroxidizable chemicals in the re- moisture and may react violently with handling, and storage of inorganic and search laboratory and published rec- a variety of substances, including wa- organic peroxy-compounds and per- ommendations for maximum storage ter. Thus, the standard iodide test for oxide-forming compounds. time for common peroxide-forming peroxides must not be used with these The relatively weak oxygen-oxygen laboratory solvents. Several solvents, water-reactive compounds.1 linkage (bond-dissociation energy of (e.g., diethyl ether) commonly used in Inorganic peroxides are used as ox- 20 to 50 kcal moleϪ1) is the character- the laboratory can form explosive re- idizing agents for digestion of organic istic structure of organic and inor- action products through a relatively samples and in the synthesis of or- ganic peroxide molecules, and is the slow oxidation process in the pres- ganic peroxides. -

Radical Initiators
Question #61073 – Chemistry – Organic Chemistry Question: List the various methods of generation of free radicals. Discuss in detail various redox sources of free radical generation. Answer: Methods of generation of free radicals Thermal Cracking At temperatures greater than 500º C, and in the absence of oxygen, mixtures of high molecular weight alkanes break down into smaller alkane and alkene fragments. This cracking process is important in the refining of crude petroleum because of the demand for lower boiling gasoline fractions. Free radicals, produced by homolysis of C–C bonds, are known to be intermediates in these transformations. Studies of model alkanes have shown that highly substituted C–C bonds undergo homolysis more readily than do unbranched alkanes. In practice, catalysts are used to lower effective cracking temperatures. Homolysis of Peroxides and Azo Compounds In contrast to stronger C–C and C–H bonds, the very weak O–O bonds of peroxides are cleaved at relatively low temperatures ( 80 to 150 ºC ), as shown in the following equations. The resulting oxy radicals may then initiate other reactions, or may decompose to carbon radicals, as noted in the shaded box. The most commonly used peroxide initiators are depicted in the first two equations. Organic azo compounds (R–N=N–R) are also heat sensitive, decomposing to alkyl radicals and nitrogen. Azobisisobutyronitrile (AIBN) is the most widely used radical initiator of this kind, decomposing slightly faster than benzoyl peroxide at 70 to 80 ºC. The thermodynamic stability of nitrogen provides an overall driving force for this decomposition, but its favorable rate undoubtedly reflects weaker than normal C-N bonds. -
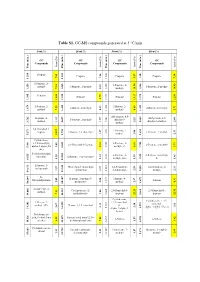
Table S1. GC-MS Compounds Generated at 5 °C/Min
Table S1. GC-MS compounds generated at 5 °C/min. 0 wt.% 10 wt.% 30 wt.% 50 wt.% GC GC GC GC Compounds Compounds Compounds Compounds Area (%) Area (%) Area (%) Area (%) Time (min.) (min.) Time Time (min.) (min.) Time (min.) Time (min.) Time Propene Propene Propene Propene 7.42 7.42 1.26 5.47 1.20 7.38 1.200 1.200 1.200 1.214 1.214 1-Propene, 2- 1-Propene, 2- methyl- 1-Propene, 2-methyl- 1-Propene, 2-methyl- 7.34 7.34 2.41 methyl- 1.245 1.245 1.245 1.259 1.259 10.54 1.246 14.90 Pentane Pentane Pentane Pentane 3.79 3.79 1.375 1.375 1.395 1.395 11.81 1.381 13.17 1.375 12.83 1-Pentene, 2- 2-Butene, 2- 2-Butene, 2-methyl- 2-Butene, 2-methyl- methyl- 7.25 0.93 methyl- 4.65 5.71 1.426 1.426 1.420 1.420 1.679 1.679 1H-Pyrazole, 4,5- Heptane, 4- 1H-Pyrazole, 4,5- 1-Pentene, 2-methyl- dihydro-5- methyl- 1.92 1.57 2.25 dihydro-5-methyl- 2.44 1.659 1.659 1.562 1.569 3.762 3.762 methyl- 2,4-Dimethyl-1- 1-Pentene, 2- heptene 2-Butene, 2,3-dimethyl- 1-Pentene, 2-methyl- 1.27 1.27 methyl- 3.08 2.33 1.653 1.653 1.653 5.671 5.671 34.57 1.750 Cyclohexane, 1,3,5-trimethyl-, 2-Pentene, 3- 2,4-Dimethyl-1-heptene 2-Pentene, 2-methyl- alpha.,3.alpha.,5.b 2.03 9.11 methyl-, €- 6.11 5.73 5.605 5.605 1.743 1.744 5.981 5.981 eta.)- 5-Aminoisoxazole 2-Pentene, 3- 2-Pentene, 3-methyl-, Spermine Ethanone, 1-cyclopentyl- 1.55 1.55 1.32 methyl-, (Z)- 1.59 €- 1.46 1.828 1.828 1.828 6.925 6.925 10.560 Ethanone, 1- 1R,2c,3t,4t-Tetramethyl- 1,3-Pentadiene, 1,4-Hexadiene, 5- cyclopentyl- 3.57 3.57 cyclohexan 0.97 2,3-dimethyl- 2.28 methyl- 2.31 2.584 2.584 2.585 10.613 10.613 10.638 N- Heptane, 2-methyl-3- Heptane, 4- Methylallylamine Toluene 2.49 2.49 methylene- 2.50 methyl- 2.97 4.31 3.697 3.697 3.697 14.047 14.047 10.684 10.684 2-Undecene, 4- Cyclopentane, (2- 2,4-Dimethyl-1- 2,4-Dimethyl-1- methyl- 7.81 7.81 methylbutyl)- 1.00 heptene heptene 5.605 5.605 22.29 22.29 5.605 12.95 14.086 14.086 14.163 Cyclohexane, Cyclohexane, 1,3,5- 2-Decene, 7- 1,3,5-trimethyl-, trimethyl- methyl-, (Z)- Hexane, 2,3,4-trimethyl- (1. -

Gfsorganics & Fragrances
Chemicals for Flavors GFSOrganics & Fragrances GFS offers a broad range of specialty organic chemicals Specialized chemistries we as building blocks and intermediates for the manufacture of offer include: flavors and fragrances. • Alkynes Over 5,500 materials, including 1,400 chemicals from natural sources, are used for flavor • Alkynols enhancements and aroma profiles. These aroma chemicals are integral components of • Olefins the continued growth within consumer products and packaged foods. The diversity of products can be attributed to the broad spectrum of organic compounds derived from • Unsaturated Acids esters, aldehydes, lactones, alcohols and several other functional groups. • Unsaturated Esters • DIPPN and other Products GFS Chemicals manufactures a wide range of organic intermediates that have been utilized in a multitude of personal care applications. For example, we support Why GFS? several market leading companies in the manufacture and supply of alkyne based aroma chemicals. • Specialized Chemistries • Tailored Specifications As such, we understand the demanding nature of this fast changing market and are fully • From Grams to Metric Tons equipped to overcome process challenges and manufacture the novel chemical products • Responsive Technical Staff that you need, when you need them. We offer flexible custom manufacturing services to produce high purity products with the assurance of quality and full confidentiality. • Uncompromised Product Quality Our state-of-the-art manufacturing facility, located in Columbus, OH is ISO 9001:2008 certified. As a U.S. based manufacturer we have a proven record of helping you take products from development to commercialization. Our technical sales experts are readily accessible to discuss your project needs and unique product specifications. -

Ionic Liquid*
Pure Appl. Chem., Vol. 83, No. 7, pp. 1391–1406, 2011. doi:10.1351/PAC-CON-10-12-03 © 2011 IUPAC, Publication date (Web): 10 June 2011 Oligomerisation of linear 1-olefins using a chlorogallate(III) ionic liquid* Martin P. Atkins1, Kenneth R. Seddon2,‡, and Małgorzata Swadźba-Kwaśny2,‡ 1PETRONAS Research Sdn Bhd, Lot 3288&3289, Off Jalan Ayer Itam, Kawasan Institusi Bangi, 43000, Kajang, Malaysia; 2QUILL, The Queen’s University of Belfast, Belfast, BT9 5AG, UK Abstract: A Lewis acidic chlorogallate(III) ionic liquid, 1-ethyl-3-methylimidazolium hepta - chlorodigallate(III), [C2mim][Ga2Cl7], was successfully used to oligomerise 1-pentene. The influence of temperature, time, catalyst concentration, and stirring rate on conversion and product distribution was modelled using a design of experiment (DoE) approach (chemo - metrics). The process was optimised for lubricant base oils production; the C20–C50 fraction (where Cn indicates the number of carbons in the oligomer) was maximised, while the heav- ier oligomer fraction (>C50) was minimised. Keywords: 1-alkenes; chemometrics; ionic liquids; linear terminal olefins; 1-olefins; oligomerisation. INTRODUCTION Oligomerisation and ionic liquids Polyalphaolefins (PAOs), used as a base for synthetic automotive lubricants (Group 4 base stock by API classification) [1], are traditionally made by oligomerisation of 1-decene to C20–C50 blend (where Cn indicates the number of carbons in the oligomer) [2] using Ziegler–Natta or Friedel–Crafts catalysts [3]. The carbon number is important, as the pour point of higher polymers (>C50) is too high, whilst the lower cut (<C20) is too volatile. 1-Decene is manufactured in a very selective manner from ethene [2]. -

Steam Cracking: Chemical Engineering
Steam Cracking: Kinetics and Feed Characterisation João Pedro Vilhena de Freitas Moreira Thesis to obtain the Master of Science Degree in Chemical Engineering Supervisors: Professor Doctor Henrique Aníbal Santos de Matos Doctor Štepánˇ Špatenka Examination Committee Chairperson: Professor Doctor Carlos Manuel Faria de Barros Henriques Supervisor: Professor Doctor Henrique Aníbal Santos de Matos Member of the Committee: Specialist Engineer André Alexandre Bravo Ferreira Vilelas November 2015 ii The roots of education are bitter, but the fruit is sweet. – Aristotle All I am I owe to my mother. – George Washington iii iv Acknowledgments To begin with, my deepest thanks to Professor Carla Pinheiro, Professor Henrique Matos and Pro- fessor Costas Pantelides for allowing me to take this internship at Process Systems Enterprise Ltd., London, a seven-month truly worthy experience for both my professional and personal life which I will certainly never forget. I would also like to thank my PSE and IST supervisors, who help me to go through this final journey as a Chemical Engineering student. To Stˇ epˇ an´ and Sreekumar from PSE, thank you so much for your patience, for helping and encouraging me to always keep a positive attitude, even when harder problems arose. To Prof. Henrique who always showed availability to answer my questions and to meet in person whenever possible. Gostaria tambem´ de agradecer aos meus colegas de casa e de curso Andre,´ Frederico, Joana e Miguel, com quem partilhei casa. Foi uma experienciaˆ inesquec´ıvel que atravessamos´ juntos e cer- tamente que a vossa presenc¸a diaria´ apos´ cada dia de trabalho ajudou imenso a aliviar as saudades de casa.