Experiment 7
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Laboratory Equipment Reference Sheet
Laboratory Equipment Stirring Rod: Reference Sheet: Iron Ring: Description: Glass rod. Uses: To stir combinations; To use in pouring liquids. Evaporating Dish: Description: Iron ring with a screw fastener; Several Sizes Uses: To fasten to the ring stand as a support for an apparatus Description: Porcelain dish. Buret Clamp/Test Tube Clamp: Uses: As a container for small amounts of liquids being evaporated. Glass Plate: Description: Metal clamp with a screw fastener, swivel and lock nut, adjusting screw, and a curved clamp. Uses: To hold an apparatus; May be fastened to a ring stand. Mortar and Pestle: Description: Thick glass. Uses: Many uses; Should not be heated Description: Heavy porcelain dish with a grinder. Watch Glass: Uses: To grind chemicals to a powder. Spatula: Description: Curved glass. Uses: May be used as a beaker cover; May be used in evaporating very small amounts of Description: Made of metal or porcelain. liquid. Uses: To transfer solid chemicals in weighing. Funnel: Triangular File: Description: Metal file with three cutting edges. Uses: To scratch glass or file. Rubber Connector: Description: Glass or plastic. Uses: To hold filter paper; May be used in pouring Description: Short length of tubing. Medicine Dropper: Uses: To connect parts of an apparatus. Pinch Clamp: Description: Glass tip with a rubber bulb. Uses: To transfer small amounts of liquid. Forceps: Description: Metal clamp with finger grips. Uses: To clamp a rubber connector. Test Tube Rack: Description: Metal Uses: To pick up or hold small objects. Beaker: Description: Rack; May be wood, metal, or plastic. Uses: To hold test tubes in an upright position. -

Corning Life Sciences Selection Guide Issue 6
www.corning.com/lifesciences Corning Life Sciences Selection Guide Issue 6 Trust Corning for Your Research For superior quality and performance in life science research tools, you can count on Corning. Our comprehensive line of advanced products and technologies is designed to meet your evolving needs in cell-based and genomics research. To learn more, please visit www.corning.com/lifesciences, or call 1.800.492.1110. Customers outside the U.S., please call 1.978.442.2200 or contact your local support office (listed below). Corning Incorporated Worldwide India Taiwan United Kingdom Life Sciences Support Offices t 91-124-235 7850 t 886 2-2716-0338 t 0800 376 8660 f 91-124-401 0207 f 886 2-2716-0339 f 0800 279 1117 Corning BV Life Sciences ASIA/PACIFIC Japan All Other European EUROPE Koolhovenlaan 12 Australia t 81 (0) 3-3586 1996/1997 Countries 1119 NE Schiphol Rijk t 61 2-9416-0492 f 81 (0) 3-3586 1291/1292 France t 31 (0) 20 659 60 51 The Netherlands f 61 2-9416-0493 Korea t 0800 916 882 f 31 (0) 20 659 76 73 f 0800 918 636 Corning Incorporated China t 82 2-796-9500 f 82 2-796-9300 Germany LATINAMERICA Life Sciences t 86 21-3222-4666 t 0800 101 1153 Brasil Tower 2, 4th Floor f 86 21-6288-1575 Singapore f 0800 101 2427 t (55-11) 3089-7419 900 Chelmsford St. Hong Kong t 65 6733-6511 The Netherlands f (55-11) 3167-0700 Lowell, MA 01851 t 852-2807-2723 f 65 6861-2913 Mexico t 800.492.1110 f 852-2807-2152 t 31 20 655 79 28 New! t (52-81) 8158-8400 t 978.442.2200 f 31 20 659 76 73 f (52-81) 8313-8589 f 978.442.2476 www.corning.com/lifesciences Online ordering now available 6/08 6M APC CLS–PSG–001-A4-REV6 Corning, Costar, CellBIND, CellCube, CellSTACK, DNA-BIND, Lambda, Octapette, Spin-X, Stripette, Stripwell, Thermowell, Transtar-96, Transwell,, 8-Pette, and 12-Pette are registered trademarks of Corning Incorporated, Corning, NY. -

Building a Morbidostat: an Automated Continuous-Culture Device For
PROTOCOL Building a morbidostat: an automated continuous- culture device for studying bacterial drug resistance under dynamically sustained drug inhibition Erdal Toprak1,2, Adrian Veres3, Sadik Yildiz2, Juan M Pedraza4, Remy Chait1, Johan Paulsson1,5 & Roy Kishony1,5 1Department of Systems Biology, Harvard Medical School, Boston, Massachusetts, USA. 2Faculty of Engineering and Natural Sciences, Sabanci University, Istanbul, Turkey. 3Health Sciences and Technology Program, Harvard Medical School, Boston, Massachusetts, USA. 4Department of Physics, Universidad de los Andes, Bogotá, Colombia. 5School of Engineering and Applied Sciences, Harvard University, Cambridge, Massachusetts, USA. Correspondence should be addressed to E.T. ([email protected]) or R.K. ([email protected]). Published online 21 February 2013; doi:10.1038/nprot.2013.021 We present a protocol for building and operating an automated fluidic system for continuous culture that we call the ‘morbidostat’. The morbidostat is used to follow the evolution of microbial drug resistance in real time. Instead of exposing bacteria to predetermined drug environments, the morbidostat constantly measures the growth rates of evolving microbial populations and dynamically adjusts drug concentrations inside culture vials in order to maintain a constant drug-induced inhibition. The growth rate measurements are done using an optical detection system that is based on measuring the intensity of back-scattered light from bacterial cells suspended in the liquid culture. The morbidostat can additionally be used as a chemostat or a turbidostat. The whole system can be built from readily available components within 2–3 weeks by biologists with some electronics experience or engineers familiar with basic microbiology. INTRODUCTION Antibiotic resistance is an important public health problem, ren- at a constant rate lower than the maximal growth rate of the popu- dering currently available drugs useless and threatening millions lation, the dilution rate of the morbidostat rdilution ≅ ∆V/(V·∆t) is of lives1–4. -

Laboratory Equipment Used in Filtration
KNOW YOUR LAB EQUIPMENTS Test tube A test tube, also known as a sample tube, is a common piece of laboratory glassware consisting of a finger-like length of glass or clear plastic tubing, open at the top and closed at the bottom. Beakers Beakers are used as containers. They are available in a variety of sizes. Although they often possess volume markings, these are only rough estimates of the liquid volume. The markings are not necessarily accurate. Erlenmeyer flask Erlenmeyer flasks are often used as reaction vessels, particularly in titrations. As with beakers, the volume markings should not be considered accurate. Volumetric flask Volumetric flasks are used to measure and store solutions with a high degree of accuracy. These flasks generally possess a marking near the top that indicates the level at which the volume of the liquid is equal to the volume written on the outside of the flask. These devices are often used when solutions containing dissolved solids of known concentration are needed. Graduated cylinder Graduated cylinders are used to transfer liquids with a moderate degree of accuracy. Pipette Pipettes are used for transferring liquids with a fixed volume and quantity of liquid must be known to a high degree of accuracy. Graduated pipette These Pipettes are calibrated in the factory to release the desired quantity of liquid. Disposable pipette Disposable transfer. These Pipettes are made of plastic and are useful for transferring liquids dropwise. Burette Burettes are devices used typically in analytical, quantitative chemistry applications for measuring liquid solution. Differing from a pipette since the sample quantity delivered is changeable, graduated Burettes are used heavily in titration experiments. -

Chemistry 2A Lab Manual Standard Operating Procedures Winter Quarter 2018
Chemistry 2A Lab Manual Standard Operating Procedures Winter Quarter 2018 Department of Chemistry University of California - Davis Davis, CA 95616 Student Name Locker # Laboratory Information Teaching Assistant’s Name Laboratory Section Number Laboratory Room Number Dispensary Room Number 1060 Sciences Lab Building Location of Safety Equipment Nearest to Your Laboratory Safety Shower Eye Wash Fountain Fire Extinguisher Fire Alarm Safety Chemicals Revision Date 12/1/2017 Preface Chemistry is an experimental science. Thus, it is important that students of chemistry do experiments in the laboratory to more fully understand that the theories they study in lecture and in their textbook are developed from the critical evaluation of experimental data. The laboratory can also aid the student in the study of the science by clearly illustrating the principles and concepts involved. Finally, laboratory experimentation allows students the opportunity to develop techniques and other manipulative skills that students of science must master. The faculty of the Chemistry Department at UC Davis clearly understands the importance of laboratory work in the study of chemistry. The Department is committed to this component of your education and hopes that you will take full advantage of this opportunity to explore the science of chemistry. A unique aspect of this laboratory program is that a concerted effort has been made to use environmentally less toxic or non-toxic materials in these experiments. This was not only done to protect students but also to lessen the impact of this program upon the environment. This commitment to the environment has presented an enormous challenge, as many traditional experiments could not be used due to the negative impact of the chemicals involved. -

Solution Bottles NEW 500Ml Square Media Bottle Erlenmeyer & Fernbach Flasks
Erlenmeyer & Fernbach Flasks Solution Bottles • Compatible with 1 bottle top filters or for use as solution storage 1 containers (Bottle Top Filters sold separately, see page 35) 1 Solution bottle neck size is 45mm 2 Curved bottle design 3 2 for easy handling 2 SOLUTION BOTTLES S, R/D F, NP, USP, PS Working Pack Qty/ Part No. Product Description volume Description Case 229781 150mL Solution Bottle 150mL 1/Bag 24 229782 250mL Solution Bottle 250mL 1/Bag 24 229784 500mL Solution Bottle 500mL 1/Bag 24 4 229785 1000mL Solution Bottle 1000mL 1/Bag 24 • Designed for mammalian suspension culture applications as well as bacterial & yeast culture NEW 500mL Square Media Bottle • Conveniently pre-sterilized 1 70mm opening for easy filling and dispensing (Erlenmeyer & Fernbach) 2 2 Over-sized vent area in cap for improved gas exchange (Erlenmeyer & Fernbach) • Ideal for media prep and storage of laboratory reagents and biological buffers 3 Fernbach flask shape increases surface area to volume ratio • Square design saves valuable shelf space and is ideal for 4 Baffled bottom improves mixing and aeration stacking, such as in a refrigerator • Plain bottom also available Part No. 229896 - Transfer Assembly • Unique individually bagged packaging allows opening one at a time ERLENMEYER & FERNBACH FLASKS S, NP, USP, A, PC 1 • PETG material reduces gas permeability Cap Bottom Max. Pack Qty/ Part No. Product Description Type Type Volume Description Case 1 Molded graduations on two sides 229850 2L Erlenmeyer Flask Vent Plain 2200mL 1/Bag 6 229855 2L Erlenmeyer Flask -

Corning Cell Culture Selection Guide Corning Is Helping to Make Your Research Possibilities Real with New and Innovative Products
Corning Cell Culture Selection Guide Corning is helping to make your research possibilities real with new and innovative products. Introduction We have recently advanced our microplate line to include many enhancmements and new products. Check out the Corning is pleased to present our Life Sciences Selection Guide. In this guide, you will find a selection of our newest, new 384 well solid, low-volume and Poly-D-Lysine micro- most innovative and most requested products. plates, as well as 384 and 1536 well microplates with generic bar codes in the Microplates Section. For more than 150 years, Corning Incorporated has leveraged its materials science and process engineering expertise to For up-to-date information on Corning Life Sciences’ collaborate closely with customers worldwide — turning comprehensive range of products and services, go to what were once only possibilities into breakthrough realities. www.corning.com/lifesciences where you can access: Q New Products Information One such reality is the Corning® Epic® System, a high- throughput label-free screening platform based on optical Q Technical Information including: biosensor technology.The system performs both biochemical - Application Notes and cell-based drug discovery applications and offers drug - Instruction Manuals developers the ability to evaluate promising new drug - Product Bulletins targets. It also allows for the observation of direct biological Q Educational Opportunities interactions not previously detectable in high-throughput Q Product Catalog Information applications. Q Product Literature For hard-to-attach cell lines, Corning offers a number of Q Complete Distributor Information modified or synthetic surfaces including Corning CellBIND® Surface, Ultra-Low Attachment, and Ultra-Web™ Surfaces. -

Organic Chemistry Laboratory Experiments for Organic Chemistry Laboratory 860-121-02 Mw 1:00-4:00
ORGANIC CHEMISTRY LABORATORY EXPERIMENTS FOR ORGANIC CHEMISTRY LABORATORY 860-121-02 MW 1:00-4:00 WRITTEN, COMPILED AND EDITED BY LINDA PAAR JEFFREY ELBERT KIRK MANFREDI SPRING 2008 TABLE OF CONTENTS SYNTHESIS OF ASPIRIN 1 MELTING POINT AND CRYSTALLIZATION 2 DISTILLATION 8 EXTRACTION 11 TLC AND CHROMATOGRAPHY 14 NATURAL PRODUCTS: ISOLATION OF LIMONENE 23 FREE RADICAL CHLORINATION 24 SN1 AND SN2 REACTIONS 27 DEHYDRATION REACTIONS 30 GRIGNARD SYNTHESIS 32 COMPUTATIONAL CHEMISTRY 36 MULTIPLE STEP SYNTHESIS 38 ORGANIC CHEMISTRY 121 EXPERIMENT 1 SYNTHESIS OF ASPIRIN FROM SALICYLIC ACID Aspirin is one of the oldest and most common drugs in use today. It is both an analgesic (pain killer) and antipyretic (reduces fever). One method of preparation is to react salicylic acid (1 ) with acetic anhydride (2) and a trace amount of acid (equation 1). O OH CH3 O COOH COOH + H + CH3COOH + (CH3CO)2O 3 4 1 2 The chemical name for aspirin is acetylsalicylic acid (3) PROCEDURE Place 3.00 g of salicylic acid in a 125 ml Erlenmeyer flask. Cautiously add 6 ml of acetic anhydride and then 5 drops of concentrated H2SO4. Mix the reagents and heat the flask in a beaker of water warmed to 80-90°C, for 10 minutes. Remove the Erlenmeyer flask and allow it to cool to room temperature. Add 40 ml of H2O and let the sample crystallize in an ice-water bath.* Filter and wash the crystals with cold water. Allow them to air dry overnight and weigh the product. What is the percent yield? One drawback to this synthetic procedure is that there is the possibility of some left over salicylic acid. -

Used for Moving Beakers Off of Hot Surfaces
Lab Equipment and Use Review Sheet Glassware Function Glassware Function Glassware Function Running Accurately Running reactions, measuring/ reactions, heating chemicals mixing mixing delivering chemicals – chemicals, volumes of easier for mixing Beaker heating Buret liquids than beakers chemicals Erlenmeyer Flask Used in Running Used to mix vacuum reactions, chemicals to heating filtration chemicals accurately mixing determine chemicals – concentration; Volumetric easier for contains exact Florence Flask Flask Filter Flask mixing than volumes beakers Used for Used for Used for mixing filtering and accurately and heating chemicals and for adding measuring running chemicals the volume reactions– without of liquids. smaller quantities Funnel Graduated spilling Test Tube than beakers and Cylinder flasks. Holding For stirring For storing Chemicals, Glass Stirring Rod chemicals small amounts covering of chemicals Watch Glass beakers during Sample Vial heating For adding Used to small evaporate amounts of Used for Evaporating liquids chemicals – lighting burner Dish usually by Plastic Pipets drops Used to add Utility Clamp – deionized used to hold water; to add objects on a solvents for ring stand. cleaning of Iron ring – Squirt Bottle beakers and used to hold other objects above glassware. a Bunsen burner flame. Ring Stand Ring stand – Beaker Tongs with Utility used to hold Clamp and various Iron Ring objects. Equipment Function Equipment Function Equipment Function Heat source in Used to grind Used to clean the chemistry chemicals into glassware lab. Uses powder natural gas. Mortar and Pestle Test tube brush/beaker Bunsen Burner brush Used to hold a Used to Used to crucible above transport hot strongly heat a flame – used Crucible tongs crucibles and substances Clay triangle in conjunction to remove Crucible and above a flame with iron ring their covers Cover Used to hold Used to handle a Used in group of test single test tube. -

Assignmentchemistry 216
EQUIPMENT CHECK LIST Chemistry 216 The following items should be stocked in your drawer. These items MUST be clean, dry, and in good condition, with no chips, cracks, or rust. Be sure equipment is COMPLETE before you declare yourself checked-in, otherwise you will be responsible for missing or broken items at the end of the semester. List any exceptions on the “List of Items for Issue" sheet. IN OUT IN OUT 1 - condenser (19/22) 1 - pasteur pipet bulb 1 - plastic funnel 1 - distilling column (19/22)condenser (19/22) 1 - glass stopper (19/22) 1 - watch glass 1 - thermometer holder, neoprene 1 - stemless funnel 1 - boiling flask, round bottom, 25mL 1 - filtervac 1 - boiling flask, round bottom, 50mL 1 - test tube clamp 1 - boiling flask, round bottom, 100mL 2 - centrifuge tubes, 15mL 1 - connecting tube, vacuum (19/22) 1 - rubber policeman 1 - connecting tube, claisen (19/22) 1 - scoopula 1 - connecting tube, 3 way (19/22) 1 - microspatula 1 - thermometer adapter (19/22) 4 - filter flasks: 1 - separatory funnel, 125mL 1 - filter flask, 25mL 1 - Büchner funnel 1 - filter flask, 50mL 4 - beakers: 1 - filter flask, 125mL 1 - beaker, 30mL 1 - filter flask, 250mL 1 - beaker, 50mL MICROGLASSWARE 1 - beaker, 100mL 1 - Hirsch funnel, plastic 1 - beaker, 250mL 1 - filter adapter 11 - Erlenmeyer flasks: 1 - column top, plastic, conical 5 - Erlenmeyer flasks, 10mL 2 - stirring rods, glass, 1 large, 1 small 2 - Erlenmeyer flasks, 25mL 4 - reaction tubes 2 - Erlenmeyer flasks, 50mL 1 - Erlenmeyer flask, 125mL 1 - Erlenmeyer flask, 250mL 2 - graduated cylinders: 1 - graduated cylinder, 10mL 1 - graduated cylinder, 25ml or 50mL Date Desk # Student's Signature AT THE BEGINNING OF THE SEMESTER: 1. -

Lab Session 10, Experiment 9: Charles'
Lab Session 10, Experiment 9: Charles’ Law The purpose of this experiment is to study the changes in the volume of a gas with changes in temperature at constant pressure. 9A Experiment 1. Use a thoroughly dried 125 mL Erlenmeyer flask for this experiment. If it is not dry, rinse the flask with a small amount of acetone or ethanol and place it upside-down on a paper towel to dry. 2. Fit the flask with a one-hole rubber stopper inserted with a short piece of dry glass tubing. Add rubber tubing to the end of the glass tube and assemble the apparatus as shown in Figure 10.1, using the125 mL flask and a 400 mL beaker. Be sure that the stopper fits tightly in the flask, the glass tubing fits tightly in the rubber stopper, and the rubber tubing fits tightly on the glass tubing. (The latter may require a hose clamp or wire winding.) Leave a 1 cm gap between the bottom of the flask and the beaker. 3. Pour water into the beaker until as much of the flask is covered as possible. 4. Using a Bunsen burner, heat the water in the beaker until it boils and then continue to heat Figure 10.1 for 5 minutes. At this point determine the temperature of the water by means of a 110 º C thermometer. Read the thermometer while its bulb is immersed in the water and record the reading as entry (c) in the data table below. Make sure the thermometer does not touch the beaker. -
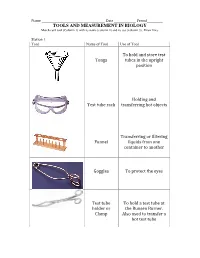
TOOLS and MEASUREMENT in BIOLOGY Tongs to Hold and Store
Name ________________________________ Date ___________ Period________ TOOLS AND MEASUREMENT IN BIOLOGY Match each tool (Column 1) with its name (column 2) and its use (column 3). Draw lines Station 1 Tool Name of Tool Use of Tool To hold and store test Tongs tubes in the upright position Holding and Test tube rack transferring hot objects Transferring or filtering Funnel liquids from one container to another Goggles To protect the eyes Test tube To hold a test tube at holder or the Bunsen Burner. Clamp Also used to transfer a hot test tube Station 2 Tool Name of Tool Use of Tool Thermometer Accurately weighs a solid Pipette Measures the temperature Accurately measures Graduated a volume of liquid Cylinder/ Measuring cylinder Triple beam Accurately measures balance/ small volumes of Electronic liquid (0.1 to 20 ml) balance Station 3 Tools Name of Tool Use of Tool Bunsen Burner With a flat bottom and a pouring beak – used to store and carry out experiments in Beaker With a flat bottom and narrow mouth – used to store and carry out experiments. Erlenmeyer Flask To conduct Or Conical flask experiment that require small volumes Hot plate Uses electricity to heat up liquids. Test Tube Uses gas and open flames to heat substances for experimentation Station 4 Tool Name of Tool Use of Tool Observation Name of Tool Use of Tool Forceps Magnify specimens up to 20 times to observe details Cavity slides View tiny specimens by magnifying up to 400 times. Specimens have to be mounted on slides. Slides To hold specimens firmly for transfer Coverslip To mount a specimen for observations under the microscope To cover the specimens Stereomicroscope on the slides before Or viewing under a Dissecting microscope.