James Walsh Postdoctoral Fellow Department of Chemistry
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cr7ni Wheels: Supramolecular Tectons for the Physical Implementation of Quantum Information Processing
magnetochemistry Review Cr7Ni Wheels: Supramolecular Tectons for the Physical Implementation of Quantum Information Processing Jesus Ferrando-Soria † School of Chemistry, University of Manchester, Oxford Road, Manchester M13 9PL, UK; [email protected]; Tel.: +34-963-544460 † Present address: Instituto de Ciencia Molecular (ICMol), Universitat de València, Paterna 46980, València, Spain. Academic Editor: Floriana Tuna Received: 11 August 2016; Accepted: 15 September 2016; Published: 21 September 2016 Abstract: The physical implementation of quantum information processing (QIP) is an emerging field that requires finding a suitable candidate as a quantum bit (qubit), the basic unit for quantum information, which can be organised in a scalable manner to implement quantum gates (QGs) capable of performing computational tasks. Supramolecular chemistry offers a wide range of chemical tools to bring together, with great control, different molecular building blocks in order to grow supramolecular assemblies that have the potential to achieve the current milestones in the field. In this review, we are particularly interested in the latest research developments on the supramolecular chemistry approach to QIP using {Cr7Ni} wheels as qubits for the physical implementation of QGs. Special emphasis will be given to the unique high degree of chemical tunability of this unique class of heterobimetallic octanuclear rings, which results in an attractive playground to generate aesthetically pleasing supramolecular assemblies of increasing structural complexity and interesting physical properties for quantum computing. Keywords: {Cr7Ni} heterobimetallic rings; quantum information processing; supramolecular chemistry; qubits; quantum gates 1. Introduction: Molecules as Qubits The physical implementation of quantum information processing (QIP) is currently a subject of intense research in chemistry, physics, materials science, and nanotechnology because of the thrilling potential technological applications that chemical and physical systems may exhibit in quantum computing [1–4]. -

N Ew Slet T Er
Electron paramagnetic resonance spectroscopy Now located in the PSI, the EPSRC National EPR Research Facility and Service is run by Profs David Collison and Eric McInnes from the School of Chemistry and has been established with £4.1M fund- ing from EPSRC, and £355K from the University of Manchester. The Facility accommodates several Bruker EPR in- struments, allowing c.w. and pulsed EPR measure- ments at frequencies between 1 (L-band) and 95 GHz (W-band), a Quantum Design magnetometer and an ODESSA instrument (built by Dr Nigel Poolton) The EPR Team from right to left: Prof Eric McInnes, Dr Stephen Sproules, Prof David Collison, Dr Floriana Tuna, that combines optically detected magnetic resonance Dr Brian Tolson, Miss Chloe Stott, Dr Nigel Poolton (absent) (ODMR) with photo-EPR (both 34 GHz; 0-4 T mag- Facilities: Frequency (GHz): 1, 4, 9.5, 24, 34, 94 net). Together these make a unique research base for Frequency Band: L-, S-, X-, K-, Q-, W- studying various types of paramagnetic species and Modes: Parallel and perpendicular at X-band materials. Temperature Range: 4.2 to 300 K all bands, to 500 K at X-band only Researchers across the country are using the Facility on a regular basis. PhD students and academics are For further details see the Facility website: encouraged to visit the Centre and to gain ―hands on‖ http://www.epr.chemistry.manchester.ac.uk/ experience. Newsletter Winter 2011/12 This newsletter consists of a combination of articles, highlighting both recent grant successes and those of a personal nature. Until further notice, items for future newsletters and/or the PSI website should be sent to [email protected]. -

The 45Th Annual International Meeting of the ESR Spectroscopy Group of the Royal Society of Chemistry
The 45th Annual International Meeting of the ESR Spectroscopy Group of the Royal Society of Chemistry The University of Manchester 25th – 29th March 2012 Contents Conference Programme 3 Information for delegates 6 Getting there 6 Map of conference venue 9 University of Manchester campus map 10 Speaker/poster presenter information 11 Internet access 11 Car parking/taxis 11 Checking out and left luggage 11 Accompanying persons 12 Free afternoon 12 Manchester city centre map 13 Conference sponsors 14 EPR @ Manchester 15 Bruker prize lecture and reception 16 JEOL student prize lectures 17 Committee of the ESR spectroscopy Group of the RSC 18 Next meeting (2013) 19 Abstracts for Talks T1‐T48 Abstracts for Posters P1‐P31 Presenting Author Index R1‐R2 Title Index R3‐R6 List of participants R7‐R14 2 Conference Programme Sunday 25th March 16.00 – 18.30 Registration Chancellors Reception 18.30 – 20.00 Dinner Chancellors Carriage Restaurant RSC Wine Reception 20.00 – 22.30 Chancellors Conservatory and bar and free bar Monday 26th March 07.30 – 08.55 Breakfast Chancellors or Luther King House or Willowbank Hotel Session 1 Chair: David Collison 08.55 – 09.00 Mark Newton Conference opening and welcome note 09.00 – 09.30 Richard Winpenny Keynote Lecture: EPR Studies of Rings and Dimers of Rings Intercluster exchange interactions and spin state switching in 09.35 – 09.50 Irina Drozdyuk copper nitroxide based molecular magnets Cu(hfac)2LR studied by EPR Quantum operations by pulsed ESR spectroscopy: Molecular 09.55 – 10.10 Shigeaki Nakazawa design for -

A Monometallic Lanthanide Bis(Methanediide) Single Molecule Magnet with a Large Energy Barrier Cite This: Chem
Chemical Science View Article Online EDGE ARTICLE View Journal | View Issue A monometallic lanthanide bis(methanediide) single molecule magnet with a large energy barrier Cite this: Chem. Sci.,2016,7,155 and complex spin relaxation behaviour† Matthew Gregson,‡a Nicholas F. Chilton,‡a Ana-Maria Ariciu,b Floriana Tuna,b Iain F. Crowe,c William Lewis,d Alexander J. Blake,d David Collison,a Eric J. L. McInnes,b Richard E. P. Winpenny*a and Stephen T. Liddle*a We report a dysprosium(III) bis(methanediide) single molecule magnet (SMM) where stabilisation of the highly magnetic states and suppression of mixing of opposite magnetic projections is imposed by a linear arrangement of negatively-charged donor atoms supported by weak neutral donors. Treatment TMS TMS TMS 2À TMS of [Ln(BIPM )(BIPM H)] [Ln ¼ Dy, 1Dy;Y,1Y; BIPM ¼ {C(PPh2NSiMe3)2} ; BIPM H ¼ À TMS {HC(PPh2NSiMe3)2} ] with benzyl potassium/18-crown-6 ether (18C6) in THF afforded [Ln(BIPM )2] [K(18C6)(THF)2] [Ln ¼ Dy, 2Dy;Y,2Y]. AC magnetic measurements of 2Dy in zero DC field show Creative Commons Attribution 3.0 Unported Licence. temperature- and frequency-dependent SMM behaviour. Orbach relaxation dominates at high temperature, but at lower temperatures a second-order Raman process dominates. Complex 2Dy exhibits two thermally activated energy barriers (Ueff) of 721 and 813 K, the largest Ueff values for any monometallic dysprosium(III) complex. Dilution experiments confirm the molecular origin of this phenomenon. Complex 2Dy has rich magnetic dynamics; field-cooled (FC)/zero-field cooled (ZFC) susceptibility measurements show a clear divergence at 16 K, meaning the magnetic observables are out-of-equilibrium below this temperature, however the maximum in ZFC, which conventionally defines the blocking temperature, TB, is found at 10 K. -

122 Structure and Bonding
122 Structure and Bonding Series Editor: D. M. P.Mingos Editorial Board: P.Day·T.J.Meyer·H.W.Roesky·J.-P.Sauvage Structure and Bonding Series Editor: D. M. P.Mingos Recently Published and Forthcoming Volumes Single-Molecule Magnets Principles and Applications and Related Phenomena of Density Functional Theory Volume Editor: Winpenny, R. in Inorganic Chemistry II Vol. 122, 2006 Volume Editors: Kaltsoyannis, N., McGrady, J. E. Non-Covalent Multi-Porphyrin Assemblies Vol. 113, 2004 Synthesis and Properties Volume Editor: Alessio, E. Principles and Applications Vol. 121, 2006 of Density Functional Theory in Inorganic Chemistry I Recent Developments in Mercury Sience Volume Editors: Volume Editor: Atwood, David A. Kaltsoyannis, N., McGrady, J. E. Vol. 120, 2006 Vol. 112, 2004 Layered Double Hydroxides Supramolecular Assembly Volume Editors: Duan, X., Evans, D. G. via Hydrogen Bonds II Vol. 119, 2005 Volume Editor: Mingos, D. M. P. Vol. 111, 2004 Semiconductor Nanocrystals and Silicate Nanoparticles Applications of Evolutionary Computation Volume Editors: Peng, X., Mingos, D. M. P. in Chemistry Vol. 118, 2005 Volume Editors: Johnston, R. L. Vol. 110, 2004 Magnetic Functions Beyond the Spin-Hamiltonian Fullerene-Based Materials Volume Editor: Mingos, D. M. P. Structures and Properties Vol. 117, 2005 Volume Editor: Prassides, K. Vol. 109, 2004 Intermolecular Forces and Clusters II VolumeEditor:Wales,D.J. Supramolecular Assembly Vol. 116, 2005 via Hydrogen Bonds I Volume Editor: Mingos, D. M. P. Intermolecular Forces and Clusters I Vol. 108, 2004 VolumeEditor:Wales,D.J. Vol. 115, 2005 Optical Spectra and Chemical Bonding in Transition Metal Complexes Superconductivity in Complex Systems Special Volume II Volume Editor: Müller, K. -

Correlating Blocking Temperatures in Single Molecule Magnets with Raman Relaxation
doi.org/10.26434/chemrxiv.7067669.v1 Correlating Blocking Temperatures in Single Molecule Magnets with Raman Relaxation Marcus J. Giansiracusa, Andreas Kostopoulos, George F. S. Whitehead, David Collison, Floriana Tuna, Richard Winpenny, Nicholas Chilton Submitted date: 10/09/2018 • Posted date: 11/09/2018 Licence: CC BY-NC-ND 4.0 Citation information: Giansiracusa, Marcus J.; Kostopoulos, Andreas; F. S. Whitehead, George; Collison, David; Tuna, Floriana; Winpenny, Richard; et al. (2018): Correlating Blocking Temperatures in Single Molecule Magnets with Raman Relaxation. ChemRxiv. Preprint. We report a six coordinate DyIII single-molecule magnet (SMM) with an energy barrier of 1110 K for thermal relaxation of magnetization. The sample shows no retention of magnetization even at 2 K and this led us to find a good correlation between the blocking temperature and the Raman relaxation regime for SMMs. The key parameter is the relaxation time (ᵰ ) at the point where switch the Raman relaxation mechanism becomes more important than Orbach. File list (1) Dy-mon_final_for ChemRxiv.pdf (1.77 MiB) view on ChemRxiv download file Correlating Blocking Temperatures in Single Molecule Magnets with Raman Relaxation Marcus J. Giansiracusa, Susan Al-Badran, Andreas K. Kostopoulos, George F. S. Whitehead, David Collison, Floriana Tuna, Richard E. P. Winpenny*, and Nicholas F. Chilton* Dedications Abstract: We report a six coordinate DyIII single-molecule magnet length to the anionic DiMeQ oxygen donors at 2.150(4) Å. The (SMM) with an energy barrier of 1110 K for thermal relaxation of trans equatorial Dy-Cl bonds are 2.681(2) Å, and the third Cl magnetization. The sample shows no retention of magnetization even ligand trans to the neutral water ligand (2.32(1) Å) has a bond at 2 K and this led us to find a good correlation between the blocking length of 2.897(8) Å. -

Electron Spin Resonance
The EPSRC National EPR/ENDOR Service is based across the Universities of Manchester, St Andrews and Cardiff. Manchester (Chemistry Department) provides facilities for CW EPR at L (ca. 1), S (4), X (9), K (24) and Q-bands (34 GHz) at ELECTRON SPIN temperatures between 4 and 300 K at all frequencies, and up to 700 K at X-band. X- and Q-band CW ENDOR facilities are available at Cardiff. St Andrews (Physics and RESONANCE RRSSxxCC Astronomy Department) offer CW EPR at 90, 180 and 270 GHz. The Service is available ROYAL SOCIETY OF CHEMISTRY to all academic researchers eligible for EPSRC funding, and is free at "point of sale". GROUP Workers in the areas of biological, pharmaceutical or materials science are welcomed. Research collaborations with international, industrial or institutional organisations are possible. Anyone interested in using the Service should contact: Dr David Collison Newsletter ([email protected]) or Dr Eric McInnes ([email protected]), Dr Graham March 2003 Smith (St Andrews, [email protected]), or Dr Damien Murphy (Cardiff, [email protected]) in the first instance. Eric McInnes ESR GROUP COMMITTEE. SpinDrift from John Walton (83 papers) with applications to protein Dr Mark E. Newton, Department of In its early years ESR spectroscopy was dynamics, membrane-peptide The ESR Group committee was elected at Chemistry, University of Warwick Dr Graham Smith, School of Physics, one of the key reference points for testing interactions, muscle fibers and the AGM held in Aberdeen on 10th April QM theory. The classic INDO semi- carbohydrates. -

School of Chemical Engineering and Analytical Sciences Catalytic Research Prof
Faculty of Sciences and Engineering Faculty of Engineering and Physical Sciences School of Chemical Engineering and Analytical Sciences Catalytic Research Prof. Chris Hardacre School of Chemical Engineering and Analytical Science [email protected] Chris Hardacre is Head of the School of Chemical Engineering and Analytical Science and Professor of Chemical Engineering, with research interests in heterogeneous catalysis, in-situ method development and ionic liquids. He has 350+ publications with an H-index of 65 and over 15,000 citations. He is a Member of the Royal Irish Academy, Fellow of the Institute of Chemical Engineering and Fellow of Royal Society of Chemistry. He has a number of awards including the inaugural Andrew Medal for catalysis and has won ~£28M research grant over the past 20 years. We are a world-leading research group working on heterogeneous catalysis and ionic liquids. We have developed a number of state-of-the-art techniques for in- situ monitoring of the systems studied and have strong links with industry. We target applications in energy, bulk, fine and pharmaceutical chemical synthesis as well as environmental protection: •Non-thermal plasma catalysis. ACS Catal., 2015, 5 956; 2014, 4, 666; •Neutron and X-ray scattering studies of catalysts and ionic liquids, Chem. Sci., 2013, 4, 3484; 2013, 4, 1270; 2011, 2, 1594; •Activating gold catalysts. ACS Catal., 2012, 2, 552; Angew. Chem. Int. Ed., 2011, 50, 8912; JACS, 2009, 131, 6973; •Electrochemical reduction of CO2. Angew. Chem. Int. Ed., 2015, 54, 14164.(Hot -
The 17Th International Conference on Molecule-Based Magnets Online
The 17th International Conference on Molecule-based Magnets Online via The University of Manchester 14 - 18 June 2021 Sponsors 2 Knowledge for your next step forward Volume 9 Number 1 7 January 2018 C h e m i c a l Pages 1-268 C h e m i c a l S c i e n c e S c i e n c e rsc.li/chemical-science Open and free, for authors and readers The Royal Society of Chemistry’s flagship journal introduces primary research in all fields to a global readership Editor-in-chief ISSN 2041-6539 EDGE ARTICLE Andrew Cooper University of Liverpool, UK Xinjing Tang et al. Caged circular siRNAs for photomodulation of gene expression in cells and mice Submit your work rsc.li/chemical-science @ChemicalScience Volume 46 Number 10 14 March 2017 D a l t o n Pages 3073-3412 Dalton Transactions T r a n s a c t i o n s An international journal of inorganic chemistry rsc.li/dalton The international journal for high quality, original research in inorganic and organometallic chemistry Fast times to publication mean rapid visibility for your work Editorial Board Chair Russell Morris University of St Andrews, UK ISSN 0306-0012 COMMUNICATION Douglas W. Stephen et al. Submit your work N-Heterocyclic carbene stabilized parent sulfenyl, selenenyl, and tellurenyl cations (XH+, X = S, Se, Te) rsc.li/dalton @DaltonTrans Get journal updates: rsc.li/alerts Registered charity number: 207890 Contents Sponsors ..................................................................................................................................... 2 Contents ..................................................................................................................................... 3 Programme ................................................................................................................................. 4 Prof. Keith Murray: Olivier Kahn Lecture ..................................................................................... 5 Prof. -
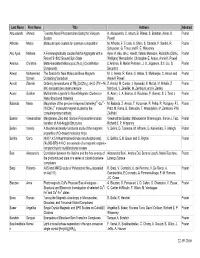
Last Name First Name Títle Authors Abstract Abouelwafa Ahmed Towards Novel Photoswitches Using the Viologen A
Last Name First Name Títle Authors Abstract Abouelwafa Ahmed Towards Novel Photoswitches Using the Viologen A. Abouelwafa, C. Anson, B. Pilawa, S. Balaban, Annie. K. Poster System Powell Affronte Marco Molecular spin clusters for quantum computation M. Affronte, F. Troiani, A. Ghirri, S. Carretta, P. Santini, R. Poster Schuecker, G. Timco and R. E. Winpenny Ako Ayuk Manase A Ferromagnetically coupled Mn19 Aggregate with a Ayuk M. Ako, Ian J. Hewitt, Valeriu Mereacre, Rodolphe Clérac, Poster Record S=83/2 Ground Spin State Wolfgang Wernsdorfer, Christopher E. Anson, Annie K. Powell Ambrus Christina Metal-templated Macrocyclic (N3O2) Coordination C.Ambrus, B. Møller Petersen, J. O. Jeppesen, S.X. Liu, S. Poster Compounds Decurtins Anwar Muhammad The Search for New Molecular Base Magnets M. U. Anwar, R. Kania, G. Abbas, S. Mukherjee, C. Anson and Poster Usman Containing Vanadium Annie K Powell Arnold Zdenek Ordering temperatures of TM3[Cr(CN)6]2.nH2O (TM – Ni, Z. Arnold, M. Cieslar, J. Kamarád, S. Maťaš, M. Mihalik, Z. Poster Mn) nanoparticles under pressure Mitróová, V. Zeleňák, M. Zentková, and A. Zentko Aromí Guillem Multidentate Ligands for Novel Magnetic Clusters or G. Aromí, L. A. Barrios, O. Roubeau, P. Gamez, S. J. Teat, J. Poster Nano-Structured Materials Ribas 5+ 2+ Balanda Maria Magnetism of the genuine bi-layered (tetrenH5) -Cu - M. Bałanda, Z. Arnold, T. Korzeniak, R. Pełka, R. Podgajny, F.L. Poster 3- [W(CN)8] molecular magnet studied by the Pratt, M. Rams, B. Sieklucka, T. Wasiutyński, M. Zentkova, P.M. complementary methods Zieliński Baskar Viswanathan Manganese, Zinc and Sodium Polyoxoantimonates: Viswanathan Baskar, Maheswaran Shanmugam, Simon J. -

Part III Chemistry Course Unit Directory
THE UNIVERSITY OF MANCHESTER School of Chemistry Programme Unit Specification Part III Chemistry Course Unit Directory 71 THE UNIVERSITY OF MANCHESTER School of Chemistry Programme Unit Specification 1. GENERAL INFORMATION Title Introductory Chemistry Unit code CM1101 Credit rating 30 credits Level Pre-requisite units Co-requisite units Member of staff responsible Prof J Christopher Whitehead 2. AIMS The programme unit aims to: To provide an introduction to the fundamental principles underlying all chemical phenomena, and establish a sound basis for the further study of all branches of Chemistry . 3. CONTENT Semester 1: Week 1 Introduction to Chemistry (Heads of the Teaching Sections) 3 lectures illustrating the place of Chemistry in the Modern World introduction to the e-learning tutorial support for this course Weeks 2-6 Foundations of Chemistry (Dr J P Day and Prof R E P Winpenny) the invention of the Periodic Table – Lavoisier to Mendeleev sub-atomic structure – Thompson to Schrödinger the quantum mechanical description of multi-electron atoms bonding and diatomic molecules common simplifications to describe bonding in polyatomic molecules Weeks 7-9 Molecular Structure, Reactivity and Functionality (Dr T W Wallace and Dr A C Regan) alkanes and cycloalkanes, conformation stereoiomerism mechanisms of reactions, electron movement and distribution curly arrows polarisation, inductive and resonance effects acidity, pKa; stability of anions haloalkanes, synthesis and uses; substitution and elimination reactions ethers, alcohols and amines alkenes, electrophilic addition; stability of carbocations alkynes; comparison with nitriles aldehydes and ketones, carboxylic acids; interconversion between alcohols, aldehydes ketones and carboxylic acids by oxidation and reduction; carboxylic acid derivatives: esters and amides Weeks 10-12 Properties of Gases. -

Esr Conf 2019.Pdf
Progress in Rapid Scan 256 scans 1048576 scans 27.2 SNR 1687.6 SNR 12 msec averaging time 51 sec averaging time Recovering Small Signals by Clean and Efficient Averaging Rapid Scan of E’ center in quartz measured with 20.5 kHz scan rate Clean white noise S/N scales with √(# averages) A million scans in 50 sec in real-time Discover more at: www.bruker.com/epr EPR Innovation with Integrity rapid-scan-ad.indd 1 11/19/2018 8:18:35 AM WELCOME On behalf of the ESR Spectroscopy Group of the Royal Society of Chemistry, welcome to the 52nd Annual International Meeting of the Group held at the Golden Jubilee Conference Hotel in Clydebank, Glasgow. The scientific sessions are headlined by our plenary and keynote speakers, as well as the recipient of the 34th Bruker Prize, Marina Bennati, and 5th Bruker Thesis Prize, Claire Motion. For younger researchers, the 22nd Annual JEOL Prize competition will run on Monday afternoon, and there are prizes for the Flash Talks on Wednesday and poster presentations. We are especially grateful to the support provided by our generous sponsors of the meeting. I hope you will have an interesting, challenging and informative time whilst in Glasgow. Stephen Sproules, Local Organizer Plenary Speakers Elena Bagryanskaya Marilena Di Valentin Russian Academy of Sciences Novosibirsk, Russia University of Padova, Italy Etienne Goovaerts David Norman University of Antwerp, Belgium University of Dundee, UK Keynote Speakers Alice Bowen Nicholas Chilton University of Oxford, UK The University of Manchester, UK Richard Cogdell