Esr Conf 2019.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ED093664.Pdf
DOCUMENT RESUME ED 093 664 SE 017 755 TITLE National Science Foundation Annual Report 1973. INSTITUTION National Science Foundation, Washington, D.C. REPORT NO NSF-74-1 PUB DATE 73 NOTE 127p. AVAILABLE FROMSuperintendent of Documents, U.S. Government Printing Office, Washington, D.C. 20402 ($2.35) EDRS PRICE MF-$0.75 HC-$6.60 PLUS POSTAGE DESCRIPTORS *Aunual Reports; Mathematics Education; *Program Descriptions; Research; *Research Projects; Science Education; Sciences; Scientific Research; Teacher Education IDENTIFIERS *National Science Foundation ABSTRACT Statistics on the allotment of funds and descriptions of activities carried out under the auspices of the National Science Foundation (NSF) in 1973 are reported. Details are provided for activities in the categories of:(1) research project support, (2). national and international programs,(3) research applications, (it): science education research and programs, and (5) science resources and policy studies. Among the appendices are a listing of staff, advisory committee, and panel members; details cf organization changes and appointments; a financial report for 1973; a description of patents resulting from NSF-supported activities; a publications list; and a list of national research centers' contractors. (RE) Ii National Science U.S. DEPARTMENT OF HEALTH, ' EDUCATION 8 WELFARE Foundation NATIONAL INSTITUTE OF EDUCATION THIS DOCUMENT HAS BEEN REPRO DUCED EXACTLY AS RECEIVED FROM THE PERSON OR ORGANIZATION ORIGIN MING IT POINTS OF VIEW OR OPINIONS Annual Report 0 STATED DO NOT NECESSARILY REPRE re1 SENT OFFICIAL NATIONAL INSTITUTE OF 90' EDUCATION POSITION OR POLICY. 1973 Letter of Transmittal Washington, D.C. DEAR MR. PRESIDENT: I have the honor to transmit herewith the Annual Report for Fiscal Year 1973 of the National Science Foundation for submission to the Congress as required by the National Science Foundation Act of 1950. -

N Ew Slet T Er
Electron paramagnetic resonance spectroscopy Now located in the PSI, the EPSRC National EPR Research Facility and Service is run by Profs David Collison and Eric McInnes from the School of Chemistry and has been established with £4.1M fund- ing from EPSRC, and £355K from the University of Manchester. The Facility accommodates several Bruker EPR in- struments, allowing c.w. and pulsed EPR measure- ments at frequencies between 1 (L-band) and 95 GHz (W-band), a Quantum Design magnetometer and an ODESSA instrument (built by Dr Nigel Poolton) The EPR Team from right to left: Prof Eric McInnes, Dr Stephen Sproules, Prof David Collison, Dr Floriana Tuna, that combines optically detected magnetic resonance Dr Brian Tolson, Miss Chloe Stott, Dr Nigel Poolton (absent) (ODMR) with photo-EPR (both 34 GHz; 0-4 T mag- Facilities: Frequency (GHz): 1, 4, 9.5, 24, 34, 94 net). Together these make a unique research base for Frequency Band: L-, S-, X-, K-, Q-, W- studying various types of paramagnetic species and Modes: Parallel and perpendicular at X-band materials. Temperature Range: 4.2 to 300 K all bands, to 500 K at X-band only Researchers across the country are using the Facility on a regular basis. PhD students and academics are For further details see the Facility website: encouraged to visit the Centre and to gain ―hands on‖ http://www.epr.chemistry.manchester.ac.uk/ experience. Newsletter Winter 2011/12 This newsletter consists of a combination of articles, highlighting both recent grant successes and those of a personal nature. Until further notice, items for future newsletters and/or the PSI website should be sent to [email protected]. -

Curriculum Vitae
CURRICULUM VITAE William Esco (W. E.) Moerner Harry S. Mosher Professor and Professor, by courtesy, of Applied Physics Department of Chemistry and Biophysics Program Stanford University, Stanford, California 94305-5080 650-723-1727 (phone), 650-725-0259 (fax), e-mail: [email protected] Education 1975 B.S. Physics Washington University (Final Honors) St. Louis, Missouri B.S. Electrical Engineering (Final Honors) A.B. Mathematics (summa cum laude) 1978 M.S. Cornell University (Physics) Ithaca, New York 1982 Ph.D. Cornell University (Physics) Ithaca, New York Thesis Topic: Vibrational Relaxation Dynamics of an IR-Laser-Excited Molecular Impurity Mode in Alkali Halide Lattices Thesis Advisor: Professor A. J. Sievers Academic Honors 1963-82 Grade Point Average of All A's (4.0) 1971-75 Alexander S. Langsdorf Engineering Fellow, Washington University 1975 Dean's Award for Unusually Exceptional Academic Achievement 1975 Ethan A. H. Shepley Award for Outstanding Achievement (university-wide) 1975-79 National Science Foundation Graduate Fellow Career Summary 2005- Professor, by courtesy, of Applied Physics 2002- Harry S. Mosher Professor of Chemistry 1998-2002 Professor of Chemistry Department of Chemistry Stanford University Multidisciplinary education and research program on single-molecule spectroscopy and quantum optics in solids, proteins, and liquids; single-molecule biophysics; and photoactive 1 polymer materials with emphasis on photorefractive polymers. Major milestones include: first room-temperature single-molecule source of single -

Curriculum Vitae
Marco Flores, Ph.D. 1 School of Molecular Sciences Arizona State University 1711 South Rural Road, Tempe, AZ 85287-1604 Phone: 480-965-8456 E-mail: [email protected] EDUCATION AND TRAINING National University of Engineering, Perú B.Sc. Physics 1993 Brazilian Center for Research on Physics, Brazil M.Sc. Physics 1996 Brazilian Center for Research on Physics, Brazil Ph.D. Physics 2000 University of California, San Diego Post-Doc Biophysics 2000-2004 PROFESSIONAL APPOINTMENTS Arizona State University, Manager at the Ultrafast Laser Facility 2018-Present Arizona State University, Research Professional and Manager of the EPR Facility 2008-Present Max-Planck Institute for Bioinorganic Chemistry, Germany, EU Research Fellow 2004-2008 Brazilian Center for Research on Physics, Brazil, Visiting Scientist 2004 Private University Antenor Orrego, Perú, Teaching Assistant 1991-1993 FELLOWSHIPS AND HONORS National Science Foundation (NSF), MRI Program, Member of EPR Review Panel 2015-2017 Wolf Foundation, Israel, Special Guest to the Ceremony of the Wolf Foundation Prize 2007 European Union/Energy Network Project (SOLAR-H), Research Fellowship 2004-2008 National Institutes of Health (NIH), Postdoctoral Fellowship 2000-2004 Ministry of Education, Brazil (CAPES), Ph.D. Fellowship 1996-2000 Ministry of Education, Brazil (CAPES), M.Sc. Fellowship 1993-1996 INVITED TALKS AND ORAL PRESENTATIONS ASU Core Facilities Symposium, Tempe, AZ 2018 George Feher Memorial Symposium, La Jolla, CA 2018 XL Encontro Nacional de Física da Matéria Condensada, Armação dos Búzios, -

Photosynthesis and the Web: 2001
Photosynthesis Research 68: 1–28, 2001. 1 © 2001 Kluwer Academic Publishers. Printed in the Netherlands. Minireview Photosynthesis and the Web: 2001 Larry Orr1 & Govindjee2,∗ 1Center for the Study of Early Events in Photosynthesis, Arizona State University, Box 871604, Tempe, AZ 85287- 1604, USA; 2Departments of Biochemistry and Plant Biology and Center of Biophysics & Computational Biology, University of Illinois, Urbana, IL 61801-3707, USA; ∗Author for correspondence (e-mail: [email protected]; fax: +1-217-244-7246) Received 15 June 2001; accepted in revised form 25 June 2001 Key words: Internet, K-12 education, Mosaic, NCSA (National Center for Supercomputing Applications), World Wide Web Abstract First, a brief history of the Internet and the World Wide Web is presented. This is followed by relevant information on photosynthesis-related web sites grouped into several categories: (1) large group sites, (2) comprehensive overview sites, (3) specific subject sites, (4) individual researcher sites, (5) kindergarten through high school (K-12) educational sites, (6) books and journals, and, 7) other useful sites. A section on searching the Web is also included. Finally, we have included an appendix with all of the web sites discussed herein as well as other web sites that space did not allow. Readers are requested to send comments, corrections and additions to [email protected]. Abbreviations: ARPA – Advanced Research Projects Agency; ASU – Arizona State University; HTML – Hy- per Text Markup Language; NCSA – National Center for Supercomputing Applications; TCP/IP – Transmission Control Protocol/Internet Protocol; UIUC – University of Illinois, Urbana-Champaign; URL – Universal Resource Locator; WWW – World Wide Web Introduction Three years ago we published a short paper detailing the then current state of photosynthesis web sites and how to find them (Orr and Govindjee 1998). -

James Walsh Postdoctoral Fellow Department of Chemistry
James Walsh Postdoctoral Fellow Department of Chemistry Northwestern University Evanston, IL 60208 phone: (847) 491-4356 email: [email protected] Current Position Assistant Professor, Department of Chemistry, University of Massachusetts Amherst (Sep 2019) Postdoctoral Fellow, Department of Chemistry, Northwestern University (2015 – Present) Advisors: Prof. Danna Freedman and Prof. Steven Jacobsen Background Postdoctoral Fellow, Aarhus University (2015) Advisor: Dr. Jacob Overgaard Ph.D. in Inorganic Chemistry, University of Manchester (2010 – 2014) Advisors: Prof. David Collison, Prof. Eric McInnes, Prof. Richard Winpenny Master’s in Chemistry, University of Manchester (2006 – 2010) Honors International Institute for Nanotechnology Outstanding Researcher Award (2017) Activities and Interests My research interests center on the use of extremely high pressure for the synthesis of completely new structures and chemical bonds. More broadly, I am interested in the use of X-ray crystallography as a tool to examine reaction mechanism in solid-state chemistry. I am a frequent user of the HPCAT and GSECARS beamlines at the APS. I collaborate closely with beamline scientists across both sectors and have averaged 8 shifts each run over the last four years. The APS is a world leader in the field of high pressure and is the source of many of the cutting-edge techniques that have since been adopted by other beamlines. This trend of origination is set to continue with the upgrade, which will position the APS at the forefront of synchrotron radiation science. The enormous increase in flux will make it the flagship of a new generation of experiments that allow for crystallographic access to unprecedented ultrafast timescales. -

General Kofi A. Annan the United Nations United Nations Plaza
MASSACHUSETTS INSTITUTE OF TECHNOLOGY DEPARTMENT OF PHYSICS CAMBRIDGE, MASSACHUSETTS O2 1 39 October 10, 1997 HENRY W. KENDALL ROOM 2.4-51 4 (617) 253-7584 JULIUS A. STRATTON PROFESSOR OF PHYSICS Secretary- General Kofi A. Annan The United Nations United Nations Plaza . ..\ U New York City NY Dear Mr. Secretary-General: I have received your letter of October 1 , which you sent to me and my fellow Nobel laureates, inquiring whetHeTrwould, from time to time, provide advice and ideas so as to aid your organization in becoming more effective and responsive in its global tasks. I am grateful to be asked to support you and the United Nations for the contributions you can make to resolving the problems that now face the world are great ones. I would be pleased to help in whatever ways that I can. ~~ I have been involved in many of the issues that you deal with for many years, both as Chairman of the Union of Concerne., Scientists and, more recently, as an advisor to the World Bank. On several occasions I have participated in or initiated activities that brought together numbers of Nobel laureates to lend their voices in support of important international changes. -* . I include several examples of such activities: copies of documents, stemming from the . r work, that set out our views. I initiated the World Bank and the Union of Concerned Scientists' examples but responded to President Clinton's Round Table initiative. Again, my appreciation for your request;' I look forward to opportunities to contribute usefully. Sincerely yours ; Henry; W. -

Interview with Amnon Yariv
AMNON YARIV (Born 1930) INTERVIEWED BY SHIRLEY K. COHEN November – December 1999 Photo by Robert Paz, CIT Public Relations ARCHIVES CALIFORNIA INSTITUTE OF TECHNOLOGY Pasadena, California Subject area Applied physics, electrical engineering Abstract Interview in three sessions in November and December 1999 with Amnon Yariv, Martin and Eileen Summerfield Professor of Applied Physics and Electrical Engineering in the Division of Engineering and Applied Science. Dr. Yariv received his BS (1954), MS (1956), and PhD (1958) from UC Berkeley. He recalls his childhood in Tel Aviv in the British Mandate of Palestine, his parents’ Polish background, and his early education, which included military training. In 1948, British occupation ends; he participates in the Israeli-Arab conflict; in 1950, leaves Israeli Army to attend the Technion, a technical university in Haifa. Emigrates to U.S. in 1951; matriculates at San Mateo Junior College; transfers to Berkeley, studies electrical engineering (control theory); switches to radio engineering, under John Whinnery, for MS; enters new field of masers for PhD. In 1959, joins group at Bell Labs under James P. Gordon working on making the first laser. Visits T. H. Maiman at Hughes Research Laboratories after Maiman produces first laser using another approach. Leaves Bell Labs to http://resolver.caltech.edu/CaltechOH:OH_Yariv_A work on lasers for Watkins-Johnson. Joins Caltech September 1964 as associate professor of electrical engineering; sets up laboratory on semiconductor lasers and another on nonlinear optics. Contacts with Roy Gould; laser work of Nicholas George. Teaches course in solid-state physics and one in laser physics called Quantum Electronics. Publishes Quantum Electronics in 1967, first text in the field. -

Electron Spin Resonance
The EPSRC National EPR/ENDOR Service is based across the Universities of Manchester, St Andrews and Cardiff. Manchester (Chemistry Department) provides facilities for CW EPR at L (ca. 1), S (4), X (9), K (24) and Q-bands (34 GHz) at ELECTRON SPIN temperatures between 4 and 300 K at all frequencies, and up to 700 K at X-band. X- and Q-band CW ENDOR facilities are available at Cardiff. St Andrews (Physics and RESONANCE RRSSxxCC Astronomy Department) offer CW EPR at 90, 180 and 270 GHz. The Service is available ROYAL SOCIETY OF CHEMISTRY to all academic researchers eligible for EPSRC funding, and is free at "point of sale". GROUP Workers in the areas of biological, pharmaceutical or materials science are welcomed. Research collaborations with international, industrial or institutional organisations are possible. Anyone interested in using the Service should contact: Dr David Collison Newsletter ([email protected]) or Dr Eric McInnes ([email protected]), Dr Graham March 2003 Smith (St Andrews, [email protected]), or Dr Damien Murphy (Cardiff, [email protected]) in the first instance. Eric McInnes ESR GROUP COMMITTEE. SpinDrift from John Walton (83 papers) with applications to protein Dr Mark E. Newton, Department of In its early years ESR spectroscopy was dynamics, membrane-peptide The ESR Group committee was elected at Chemistry, University of Warwick Dr Graham Smith, School of Physics, one of the key reference points for testing interactions, muscle fibers and the AGM held in Aberdeen on 10th April QM theory. The classic INDO semi- carbohydrates. -
Download Trial Versions of Our Software
2012 volume 22 number 1 news letter The Publication of the International EPR (ESR) Society newsepr letter www.epr-newsletter.ethz.ch Officers of the international ePr (esr) society The official publication of the International EPR (ESR) Society is supported by the Society, by corporate and President secretArY other donors, the Zavoisky Physical-Technical Institute of the Russian Academy of Sciences, Kazan, Russian seigo Yamauchi sushil K. Misra Federation, and the Swiss Federal Institute of Technology, Institute of Multidisciplinary Research for Concordia University, Zürich, Switzerland. Advanced Materials (IMRAM), 1455 de Maisonneuve Boulevard West, Tohoku University, Montreal (Quebec), H3G 1M8, Canada Katahira-2-1-1, phone: 514-848-2424 ext. 3278, fax: 514-848-2828 editOr Aobaku, Sendai 980-8577, Japan e-mail: [email protected] Laila V. Mosina phone: 81-22-217-5617, fax: 81-22-217-5616 web: physics.concordia.ca/faculty/misra.php Zavoisky Physical-Technical Institute e-mail: [email protected] Russian Academy of Sciences treAsurer Kazan, Russian Federation Vice Presidents tatyana i. smirnova [email protected] Americas North Carolina State University, AssOciAte editOrs Lawrence Berliner Department of Chemistry, Candice S. Klug Department of Chemistry and Biochemistry, Campus Box 8204, Raleigh, NC 27695-8204, USA Medical College of Wisconsin University of Denver, phone: (919) 513-4375, fax: (919) 513-7353 Milwaukee, WI, USA 2090 E. Iliff Ave, Denver, CO, OR 80208 USA e-mail: [email protected] [email protected] phone: 303-871-7476, fax: 303-871-2254 Hitoshi Ohta e-mail: [email protected] ImmediAte PAst President Molecular Photoscience Research Center, web: www.du.edu/chemistry/Faculty/lberliner.html Jack H. -
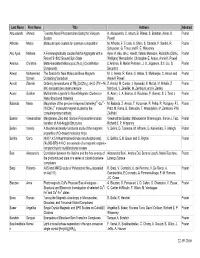
Last Name First Name Títle Authors Abstract Abouelwafa Ahmed Towards Novel Photoswitches Using the Viologen A
Last Name First Name Títle Authors Abstract Abouelwafa Ahmed Towards Novel Photoswitches Using the Viologen A. Abouelwafa, C. Anson, B. Pilawa, S. Balaban, Annie. K. Poster System Powell Affronte Marco Molecular spin clusters for quantum computation M. Affronte, F. Troiani, A. Ghirri, S. Carretta, P. Santini, R. Poster Schuecker, G. Timco and R. E. Winpenny Ako Ayuk Manase A Ferromagnetically coupled Mn19 Aggregate with a Ayuk M. Ako, Ian J. Hewitt, Valeriu Mereacre, Rodolphe Clérac, Poster Record S=83/2 Ground Spin State Wolfgang Wernsdorfer, Christopher E. Anson, Annie K. Powell Ambrus Christina Metal-templated Macrocyclic (N3O2) Coordination C.Ambrus, B. Møller Petersen, J. O. Jeppesen, S.X. Liu, S. Poster Compounds Decurtins Anwar Muhammad The Search for New Molecular Base Magnets M. U. Anwar, R. Kania, G. Abbas, S. Mukherjee, C. Anson and Poster Usman Containing Vanadium Annie K Powell Arnold Zdenek Ordering temperatures of TM3[Cr(CN)6]2.nH2O (TM – Ni, Z. Arnold, M. Cieslar, J. Kamarád, S. Maťaš, M. Mihalik, Z. Poster Mn) nanoparticles under pressure Mitróová, V. Zeleňák, M. Zentková, and A. Zentko Aromí Guillem Multidentate Ligands for Novel Magnetic Clusters or G. Aromí, L. A. Barrios, O. Roubeau, P. Gamez, S. J. Teat, J. Poster Nano-Structured Materials Ribas 5+ 2+ Balanda Maria Magnetism of the genuine bi-layered (tetrenH5) -Cu - M. Bałanda, Z. Arnold, T. Korzeniak, R. Pełka, R. Podgajny, F.L. Poster 3- [W(CN)8] molecular magnet studied by the Pratt, M. Rams, B. Sieklucka, T. Wasiutyński, M. Zentkova, P.M. complementary methods Zieliński Baskar Viswanathan Manganese, Zinc and Sodium Polyoxoantimonates: Viswanathan Baskar, Maheswaran Shanmugam, Simon J. -

EPR @ Warwick 14
The 46th Annual International Meeting of the ESR Spectroscopy Group of the Royal Society of Chemistry The University of Warwick 7th – 11th April 2013 Contents Conference Programme 1 Information for delegates 6 Getting there and How to find 6 The University of Warwick campus map 8 Speaker/poster presenter information 9 Checking in/out 9 Internet access 9 Car parking/taxis 10 Accompanying persons 11 Free afternoon 11 Conference sponsors 13 EPR @ Warwick 14 Bruker prize lecture and reception 15 JEOL student prize lectures 16 Committee of the ESR spectroscopy Group of the RSC 17 List of participants 18-22 Next meeting (2014) 23 Abstracts for Talks Abstracts for Posters The 46th International Meeting of the ESR Spectroscopy Group, Warwick 2013 The 46th International Meeting of the ESR Spectroscopy Group, Warwick 2013 Conference Programme: The 46th Annual International Meeting of the ESR Spectroscopy Group, 7th – 11th April 2013 Sunday 7th April 16.00 – 18.00 Registration Conference Reception (Collection of Room Keys) 18.30 – 20.00 Dinner Rootes Restaurant 20.00 – 22.30 RSC Reception The Bar/Bar Fusion, Rootes Building Monday 8th April 07.30 – 08.55 Breakfast Rootes Restaurant Session 1 Chair: Gavin Morley Physics Lecture Theatre 08.55 – 09.00 Mark Newton Welcome and Conference Opening 09.00 – 09.40 K1 Joerg Wrachtrup Keynote Lecture: Sensing nuclear spins at the nanoscale Tuning Molecular Magnets for Quantum Information 09.40 – 10.00 O1 Amy Webber Processing 10.00 – 10.20 O2 Floriana Tuna Single Molecule Magnetism in f-Block Metal Complexes